High-nickel layered Li[NixCoyMn1-x-y]O2 (NCM, x≥0.5), which has relatively low cost, high specific capacity and excellent comprehensive performance, is currently the most promising high-energy density lithium-ion battery The cathode material can achieve a single charge range of more than 300 miles for electric vehicles. Conventional polycrystalline high nickel layered cathodes (PCNCs) are usually designed as micron-scale secondary particles agglomerated by nano-scale primary particles to obtain high packing density and low specific surface area. However, the polycrystalline NCM cathode material will undergo H2→H3 phase transition under high state of charge, resulting in the dramatic anisotropic shrinkage/expansion of the c-axis of the crystal lattice, which promotes the generation and accumulation of a large amount of mechanical stress inside the particle, and after long-term cycling lead to the formation of intergranular microcracks. Intergranular cracks are the main reason for the degradation of their electrochemical performance; this is because the electrolyte will penetrate into the active particles along the cracks, aggravating the parasitic side reactions between the highly active Ni3+/4+ in the electrode material and the electrolyte, and then causing the surface A series of detrimental consequences such as interfacial irreversible phase transition, lattice oxygen loss and thermal runaway.
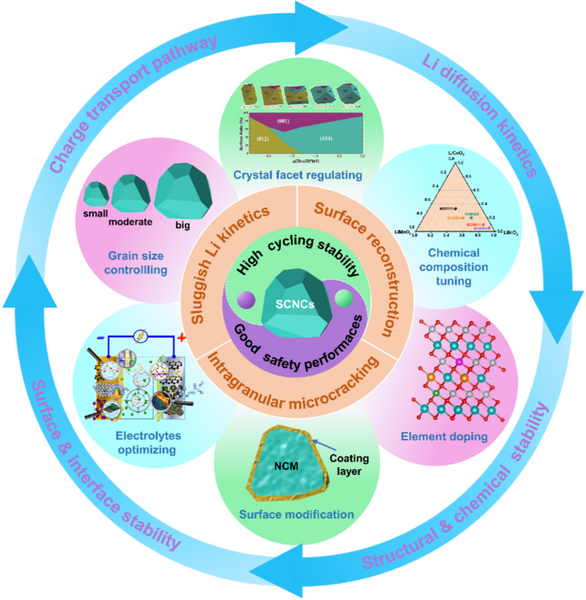
In recent years, single-crystal high-nickel layered cathodes (SCNCs) have attracted extensive attention due to their advantages such as eliminated primary grain boundaries, consistent and continuous lattice orientation, ordered crystal plane arrangement, and low specific surface area. Effectively alleviate the generation of particle microcracks. Compared with PCNCs with typical secondary particle morphologies, SCNCs have micron-sized intact primary particles, which are beneficial to enhance structural stability, alleviate parasitic side reactions between electrode/electrolyte interfaces, and further improve cycling and thermal stability. , suppressing the evolution of the gas. In addition, the void-free and integrated structure of the single crystal also has high mechanical strength, which can further increase the compaction density and thus the energy density. However, various reported works currently focus on the trial-and-error synthesis and modification of SCNCs, as well as the comparison of electrochemical performance and lithium storage mechanism with PCNCs. There is still a lack of systematic analysis and summary to reveal the key challenges of SCNCs, Structural and electrochemical performance advantages and controversies, and modification strategies.
1. Structural and electrochemical properties of SCNCs and comparison with PCNCs
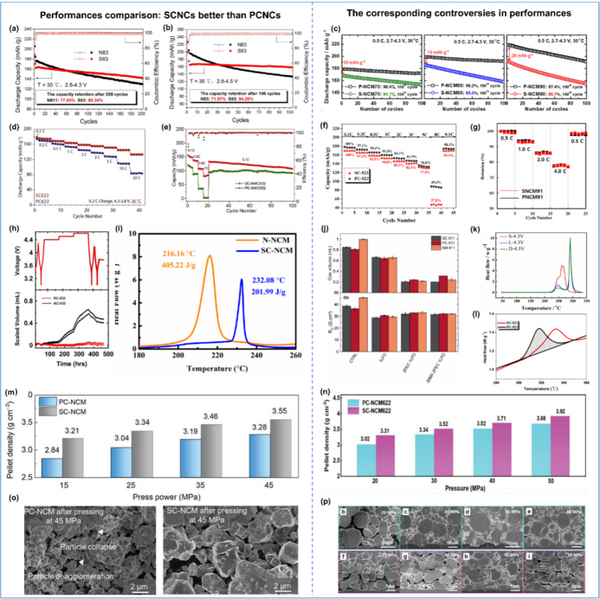
Cycling stability: It is generally believed that SCNCs have better cycling stability than PCNCs, because SCNCs have a unique integrated single crystal structure that eliminates the randomly oriented primary grain boundaries inside the particles, high mechanical strength, and small specific surface area. Particle crack formation and surface/interface side reactions can be significantly suppressed, further mitigating irreversible surface degradation. However, there are also a few literatures reporting that the cycling stability of SCNCs is inferior to that of PCNCs, which attribute this phenomenon to the larger primary particle size of SCNCs, which leads to a longer Li+ diffusion path, which leads to non-uniformity during cycling. Lithium concentration distribution. This trend will be further exacerbated with increasing Ni content and current density in the cathode, resulting in the coexistence of two phases, H2 and H3 within a single particle, and the resulting non-uniform strain and stress can create structural defects that further constrain Li+ diffusion kinetics and ultimately lead to rapid capacity decay during long-term cycling.
Rate capability: Generally speaking, SCNCs have a long Li+ diffusion path due to their micron-scale large primary particles, which makes the Li+ diffusion kinetics relatively slow, which will lead to the deterioration of their rate capability. However, in most of the literatures, the rate performance of SCNCs is better than that of PCNCs, and only a few literatures report that SCNCs have lower or similar rate performance and charge transport kinetics than PCNCs. This is mainly attributed to the advantages of the unique single crystal structure of SCNCs, including submicron size, consistent lattice orientation, ordered and periodic crystal plane arrangement, and good mechanical integrity. In addition, SCNCs have well-dispersed primary particles, which can be uniformly encapsulated by the conductive agent, which is beneficial to the fast transport of interfacial electrons between adjacent particles. In particular, SCNCs also show better rate performance when applied to all-solid-state batteries, with 6–14 times higher Li+ diffusion coefficients than PCNCs, due to the fact that the individual particles of SCNCs have eliminated grain boundaries and thus have continuous Li+ diffusion path.
Gas evolution and thermal safety: In general, the thermal activity and interfacial parasitic side reactions between high-Ni electrodes and electrolytes are intensified due to the increased Ni content in the lattice, which is associated with a large number of high catalytic and oxidative effects during charging. The active Ni3+/Ni4+ are closely related. This can further lead to serious safety issues, including gas generation and thermal runaway. Especially for PCNCs, during long-term cycling, intergranular cracking will inevitably occur due to fatigue strain caused by the dramatic shrinkage/expansion of the lattice, which will lead to more active surface exposure, further aggravating gas generation and Thermal runaway. However, SCNCs with high mechanical integrity and low specific surface area can effectively suppress particle cracking and interfacial parasitic reactions, which are expected to alleviate the gas generation and thermal runaway problems deeply rooted in high-Ni cathodes. Nonetheless, there are still many differences and debates on the gas evolution and thermal stability of SCNCs in the reported literature; in order to better compare the gas evolution and thermal safety of SCNCs and PCNCs, it is recommended to use conditions as close as possible to the actual operating conditions. Carry out tests such as full cells with high electrode loadings, sealed and electrolyte wetted environments, etc.
Mechanical stability and compaction density: Compared with PCNCs, SCNCs have higher mechanical reliability and compaction density, thanks to their micron-scale primary particles, consistent lattice orientation, and eliminated internal voids, which further It can improve its volume energy density.
2. Challenges faced by SCNCs
Although SCNCs possess many advantages over PCNCs, there are still many challenges, such as immature and complex synthetic routes, sluggish Li+ diffusion kinetics, microcrack evolution under high voltage and/or high current operation, irreversible surface weighting structure, etc., which further leads to the deterioration of electrochemical performance and hinders the commercialization process.
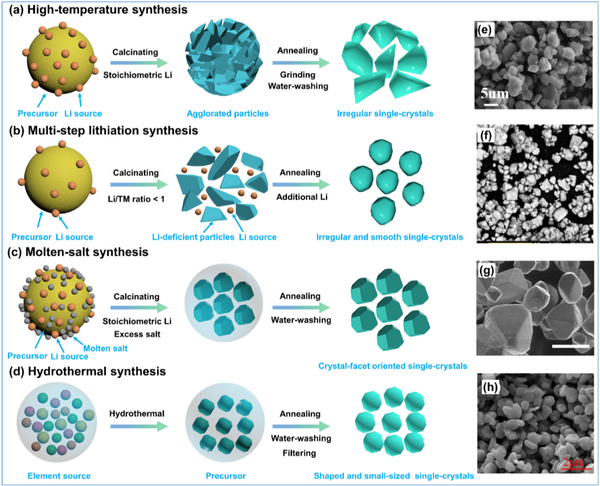
Figure 2. Schematic diagram of the four main synthetic routes of SCNCs and their corresponding SEM images: a) high-temperature synthesis, b) multi-step lithiation synthesis, c) molten salt synthesis, and d) hydrothermal synthesis.
2.1 Immature and complex synthetic routes
Unlike the well-established and standard synthetic routes for PCNCs, the synthesis of SCNCs is more complex and expensive because single crystals have higher requirements on morphology, size distribution, and particle dispersibility. Although many preparation methods for SCNCs have been reported, there is currently no general synthetic standard. As shown in Figure 2, the synthetic routes of SCNCs are mainly divided into four types: high temperature synthesis, multi-step lithiation synthesis, molten salt synthesis, and hydrothermal synthesis. In addition, synthetic methods such as spray pyrolysis, rheology-phase transition, sol-gel, etching, hydrogen peroxide-assisted synthesis, and all-dry synthesis have been reported for the preparation of SCNCs. These synthetic routes have more or less problems such as complex process, high energy consumption, high equipment requirements, high synthesis cost, and low yield.
2.2 Slow Li+ diffusion kinetics
SCNCs have a long Li+ diffusion path due to their micron-sized large primary particles, which makes the Li+ diffusion kinetics relatively slow. This may promote the uneven distribution of Li+ concentration inside the single crystal grains during the charge-discharge process, which further generates uneven structural stress, leads to the formation of intragranular microcracks, and exacerbates surface/interface failure and capacity fading. Especially at low SOC (∼3.5V) and high SOC (∼4.17V), SCNCs have more severe kinetic barriers to Li+ diffusion. Among them, the slow Li diffusion kinetics at low SOC may be related to the high activation energy caused by few Li vacancies, while at high SOC may be caused by the dramatic shrinkage of the lattice c-axis during the H2-H3 phase transition.

