Taking performance snapshots in weeks instead of years
A speaker at the BATTERIES 2013 conference in Nice, France, flashed charts on the screen that showed continuously rising energy densities. When the audience asked the presenter: “Do you believe in these predictions?” the self-conscious speaker replied in a strong Chinese accent, “no.” A subdued laugher arose. The battery industry does not foresee significant improvements in energy density in the near future.
After the 2008 callback when Li-ion disassembled in consumer products, safety gained added attention and batteries became safer. With the advent of the electric vehicle, longevity is moving to the forefront and experts begin exploring what causes batteries to fail. While a two-to-three year battery life with 500 cycles is acceptable for laptops and mobile phones, the eight-year warranty of an EV appears short when considering that a replacement battery carries the price of a new compact car. If the life of the battery could be extended to, say, 20 years, then driving an EV would be justified even if the initial investment is high. Driving a fancy EV, such as the Tesla Model-S, may be more novelty than utility.
In October 2012, Leaf owners in California and Arizona sued Nissan, claiming that the vehicles have a design defect that causes them to prematurely lose battery life and driving range. Heat when driving in a hot climate was blamed. The battery in the Leaf has no active thermal management to keep the cells cool. This omission was given as the reason why the battery would lose 27.5 percent of its capacity after one-to-two years of ownership.
EV manufacturers choose battery systems very carefully. The selection begins by picking cells that are optimized for longevity rather than high specific energy. Batteries deployed for industrial applications are normally larger and heavier than those used in consumer goods for the same ampere/hour.
Nissan selected a manganese-based Li-ion because of good performance. Batteries must go through strenuous life cycle testing and to beat the clock, the test protocol often mandates a rapid charge of 1.5 C (less than one hour) and a discharge of 2.5C (20 minutes) under a soaring temperature of 60°C (140°F). Even under these conditions, the battery may lose only 10 percent after 500 cycles, which represents one to two years of driving. This would emulate driving an EV through the heat of a biblical hell, leaving rubber marks for aggressive driving, and still come out with a battery boasting 90 percent capacity. Why then would the Leaf under more reasonable conditions drop the capacity by so much?
Field failures only come to light after the product had been in use for a few years. Professor Jeff Dahn at Dalhousie University knows this and together with his colleagues developed coulombic efficiency (CE), a method that defines the efficiency with which electrons are transferred in an electrochemical system.
During charge, lithium gravitates to the graphite anode (negative electrode) and the voltage potential changes. Removing the lithium again during discharge does not reset the battery fully. A film consisting of lithium atoms forms on the surface of the anode called solid electrolyte interface (SEI). Composed of lithium oxide and lithium carbonate, the SEI layer grows as the battery cycles. The film gets thicker and eventually forms a barrier that obstructs interaction with graphite.
The cathode (positive electrode) develops a similar restrictive layer known as electrolyte oxidation. Dr. Dahn stresses that a voltage above 4.10V/cell at high heat causes this, a demise that can be more harmful than cycling. The longer the battery stays in this condition, the worse the degradation gets. The build-up can result in a sudden capacity loss that is difficult to predict by cycling alone. This phenomenon had been known for some years but measuring the coulombic efficiency can verify these effects in a more scientific and systematic manner.
CE measures both changes: the lithium lost due to SEI growth on the anode and electrolyte oxidation at the cathode. The results can be used to rank the life expectancy of a battery by quantifying the parasitic reaction. The CE of a perfect battery would be 1.000,000. If this were the case, Dr. Dahn says, the Li-ion battery would last for ever. An excellent coulombic efficiency is 0.9999, a level some LCO (Lithium Cobalt Oxide2) reach. By far the best Li-ion batteries in terms of CE are those using Lithium Titanate (LTO) as anodes. They have the potential of delivering 10,000 cycles. The negatives are high cost and reduced specific energy.
CE readings vary with temperature and charge rate, also known as C-rate. As the cycle time gets longer, self-discharge comes into effect and CE drops (gets worse). Electrolyte oxidation at the cathode causes self-discharge. Li-ion loses about two percent per month at 0C (32F) and half-charge; up to 35 percent at 60C (140F) when fully charged. Table 1 provides data of the most common Li-ion system. CE is described as excellent, good moderate and poor taken at 30°C (86°F).
|
Chemical name |
Material |
Coulombic Efficiency1 |
Notes |
|---|---|---|---|
|
Lithium Cobalt Oxide2 (LCO) |
LiCoO2 (60% Co) |
Good, only slight drop at 50–60°C |
High capacity, limited power; fragile, mobile phone, laptop |
|
Lithium Manganese Oxide2 (LMO) |
LiMn2O4 |
Poor, CE is low, drops further at 40°C |
|
|
Lithium Iron Phosphate2 (LFP) |
LiFePO4 |
Moderate, CE drops at 50–60°C |
|
|
Lithium Nickel Manganese Cobalt Oxide2 NMC |
LiNiMnCoO2 (10–20% Co) |
Good, small drop at 60°C |
|
|
Lithium Nickel Cobalt Aluminum Oxide2 (NCA) |
LiNiCoAlO2 (9% Co) |
N/A |
Electric powertrain (Tesla Model S), grid storage |
|
Lithium Titanate3 (LTO) |
Li4Ti5O12 |
Excellent |
Very durable but expensive and low specific energy |
Table 1: Most commonly used Li-ion with Coulombinc Efficiency rated in excellent, good, moderate and poor. Battery manufacturers may one day specify CE in a number.
1 Taken at C/20 and 30°C (86°F). (20h charge & discharge); 2 Cathode material; 3 Anode material
Lithium-ion has improved and credit goes to electrolyte additives. Each cell has several additives and manufacturers keep the combinations a secret. Additives lower internal resistance by reducing corrosion, decrease gassing, speed up manufacturing by fine-tuning the wetting process, and improve low and high temperature performance. Adding 1–2 percent Vinylene Carbonate improves SEI on the anode, limits electrolyte oxidation at the cathode and enhances the CE readings. Other additives provide added benefits and one asks: “Can these chemicals interact with each other?” As a patient taking multiple medications must inform the doctor before additional pills can be prescribed, similar conditions may also exist with batteries. CE discovers possible interference in weeks rather than having to wait for years for symptoms to develop.
To examine the correlation between CE and longevity, Dalhousie works with battery manufacturers, including E-One Moli in Vancouver. The test bed consisted of 160 cells, four of each type. E-One Moli provided 80 cells with their own secret sauce; Dalhousie specified the other 80 electrolyte samples. All ingredients were carefully documented, except those provided by the cell manufacturer; these are kept as a top secret.
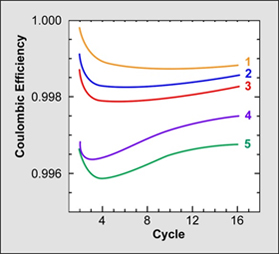
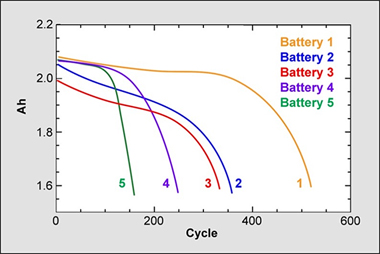
Dalhousie identified five batteries of interest. Table 2 shows the CE of these five samples with values ranging from 0.9960 to 0.9995; Table 3 demonstrates the test results when cycled to death. To Dalhousie’s anticipation and satisfaction, CE harmonized well with the cycle count. Batteries with high CE lasted the longest; those with low CE values were the first to die.
|
Table 2: Coulombinc Efficiency. Five experimental batteries are tested for coulombic efficiency. A higher CE provides a longer life. Courtesy of the Dalhousie University |
|
|
|
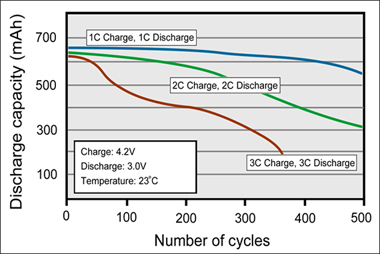
Battery wear-and-tear also includes structural degradation that can be captured with traditional cycle testing. Dr. Dahn calls this type of testing the “sausage machine.” While measuring coulombic efficiency, in which Dalhousie is leading, helps in the development of batteries by giving a snapshot assessment of additives; the old sausage machine does the verification thereafter. Figure 4 demonstrates capacity loss caused by the structural degradation of an older Li-ion when cycled at a 1C, 2C and 3C. The rapid loss of capacity at higher C-rates may be lithium planting at the anode due to rapid charging.
|
Figure 4: Cycle performance of Li-ion with 1C, 2C and 3C charge and discharge Moderate charge and discharge currents reduce structural degradation. This applies to most battery chemistries. |
|
Dalhousie’s coulombinc efficiency has gained the interest of device manufacturers, including healthcare and makers of EVs. Tesla cars use the 18650 because the cell is readily available at a low price. This was an unlike choice for the Tesla Roadster, the first EV by Tesla, as the cell was designed to power cameras, laptops, consumer products, medical devices and e-bikes. Perhaps unknown to Elon Musk, the founder of Tesla Motor, cobalt-based lithium-ion has a high (good) CE reading that adds to longevity if used carefully, the lack of robustness was solved by oversizing the pack.
Today, the Tesla Model-S uses Lithium Nickel Cobalt Aluminum Oxide (NCA), a chemistry that has high specific energy, high specific power and a long cycle count, but it costs a bit more. Tesla is also super-sizing the NCA to reduce stress. The batteries of the Model S-60 and S-85 are so large that they can operate at a C-rate of only 0.25C (C/4), even at highway speed. This allows Tesla to focus on high energy density for maximum runtime and longevity; power density is less important. The negative is increased energy consumption due to a heavier vehicle and a higher battery price.
The manganese-based Li-ion batteries of the Nissan Leaf have excellent lab result but what may have been overlooked is the damage done when keeping the battery at high voltage and high temperature. As the CE tests reveal, these two conditions can cause more damage than mere cycling, especially with LMO (Lithium Manganese Oxide2). The NMC (Lithium Nickel Manganese Cobalt Oxide2) is better and only shows a worsening CE performance above 50°C (123°F). The good news is that the Leaf battery is robust and will perform well in most parts of the world.
Henrik Fisker chose LFP (Lithium Iron Phosphate2) by A123, also a robust system when cycled in the laboratory but it has less favorable CE readings when operated above 50°C (123°F). While the demand for the Tesla Model-S exceeds production capability, the equally stunning Fisker sports car is no longer produced.
A successful EV market will eventually replace the 18650 with a larger format prismatic or pouch cell. The price per kWh will drop and the advantage of the 18650 will varnish. Good performance, high volume and multi-sourcing made the 18650 a frontrunner in lithium-ion.
Summary
The four suspected renegades that are responsible for capacity loss and the eventual end-of-life of the Li-ion battery are:
- Mechanical degradation of electrodes or loss of stack pressure in pouch-type cells. Careful cells design and correct electrolyte additives minimize this cause.
- Growth of the solid electrolyte interface (SEI) on the anode that forms a barrier and obstructs the interaction with graphite.
- Formation of the electrolyte oxidation at the cathode that may lead to sudden capacity loss. Keeping the cells at a high voltage and at an elevated temperature promotes this phenomenon.
- Lithium-plating on the surface of the anode caused by high charging rates. (The elevated capacity loss at higher C-rates in Figure 4 could be caused by this.)
Price and longevity will dictate how far the battery can go and the EV sets the upper boundary. With current technologies, deploying batteries for trains, ships and airplanes makes little sense. Competing against mighty oil with a net calorific value that is 100 times higher than that of the battery is tough. Conversely, petroleum cannot touch the battery that is clean, quiet, small, and provides an immediate startup with the flick of a switch. Incremental battery improvements will eventually secure more of what is so strongly entrenched in the seemingly endless flow of cheap fossil fuel.
References
With thank Dr. Jeff Dahn, Professor of Physics and Chemistry, Dalhousie University for his editing efforts. Dr. Dahn is recognized worldwide as a distinguished scientist in the field of advanced lithium batteries. He is one of the pioneering developers of the lithium-ion battery, authored 560 refereed journal papers and issued or filed 61 patented inventions. Dr. Dahn is one of the most prolific authors in the Journal of the Electrochemical Society and has one of the most-cited papers in the journal.

