All-solid-state lithium metal batteries have broad application prospects in the fields of energy storage, electric vehicles, and portable electronic devices due to their significantly improved safety and energy density. However, the interface problem is still the key to limiting the performance of all-solid-state lithium batteries. The high-impedance interfacial layer caused by the instability of the electrode-electrolyte interface will dominate the internal resistance of the battery, especially for the interface formed by the sulfide electrolyte and the strongly reducing lithium anode. Numerous experimental and theoretical studies, such as in-situ XPS, in-situ Raman, in-situ TEM, and first-principles calculations, have shown that sulfide electrolytes have a narrow electrochemical window and will generate Li2S, Li3P, and LiX when in contact with lithium metal (for Li6PS5X, X = Cl, Br, I), Li-Ge alloys (for Li10GeP2S12) and other products. However, the existing research mainly focuses on the precise determination of the interface reaction products, while the nanostructure of the interface layer, which is closely related to the battery performance, is rarely studied, which largely determines whether the interface can achieve good passivation.
【Introduction to Achievements】
Recently, the team of Professor Zhang Xing of Tsinghua University and the team of Professor Zhu Lingyun of Guilin Electric Power Institute have used multi-scale characterization methods such as Raman spectroscopy, cryo-transmission electron microscopy, and ab initio molecular dynamics simulations to study single lithium dendrites at different temperatures. Nanostructure and evolution of sulfide electrolyte interfacial layers. The study found that the interfacial layer formed by metal lithium and sulfide electrolyte at room temperature is single crystal Li2S, which has a good passivation effect on the interface; while at 60 ℃, the interfacial layer is significantly thickened polycrystalline Li2S, and the reaction process is accompanied by disorder - an ordered phase transition. Numerous grain boundaries, dislocations, and increased thickness within the interfacial layer at high temperatures lead to ultra-high interfacial resistance. Reaction kinetics simulations further elucidate the atomic-scale structural evolution of the metallic lithium-sulfide electrolyte interface and propose stable coating materials that are expected to completely suppress interfacial reactions. The related research results were published in the top international journal ACS Energy Letters as "Nanostructure of interphase layer between a single Li dendrite and sulfide electrolyte in all-solid-state Li batteries".
【main content】
1. Evolution of the impedance spectrum and determination of the composition of the interface layer
The interfacial reaction process of lithium metal and sulfide electrolyte Li6PS5Cl (LPSCl) can be reflected from the evolution of in situ impedance spectroscopy. Figure 1a–c show the electrochemical impedance spectra and the evolution of the corresponding interfacial impedance for Li|LPSCl|Li cells left standing at different temperatures for 120 h. It can be found that the interfacial impedance of the battery kept at -20 °C fluctuates and rises slowly. The interfacial resistance increased from 4 Ω to ~13 Ω within 120 h of the cell at 25 °C. At the same time, the slope of the interfacial resistance growth gradually decreases, so it can be inferred that the reaction is diffusion-controlled at room temperature. For the cells left at 60 °C, the interfacial resistance increased by 2 orders of magnitude, indicating a significant enhancement of side reactions. At the same time, the slope of the interfacial resistance increases first and then decreases. It can be inferred that the reaction at high temperature is controlled by reaction at the early stage, and then turned to be controlled by diffusion. In addition, the interface resistance (204 Ω) at 60 °C is 14 times higher than that of the bulk material (14.4 Ω), so the control step of ion transport at high temperature is not in the bulk phase, but at the interface.
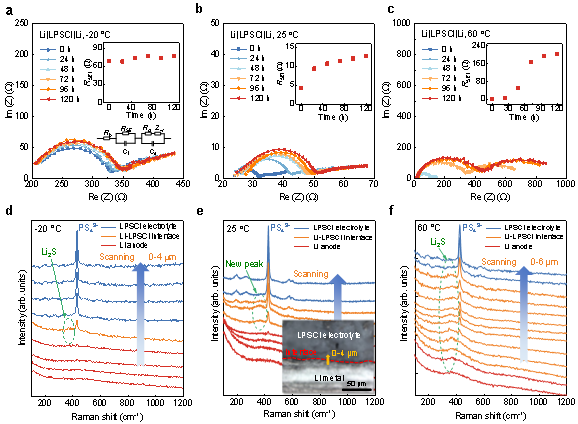
To determine the composition of the interfacial layer, the researchers used Raman spectroscopy to test Li-LPSCl cross-sections that were left standing for 120 h. Figure 1d–f show the line scan Raman spectra of the Li-LPSCl interface at different resting temperatures. The characteristic Raman peak at 425 cm-1 is attributed to the LPSCl electrolyte and is caused by the symmetrical stretching vibration of the P-S bond in the PS43-anion group. Comparing the Raman spectra of Li-LPSCl interface and LPSCl electrolyte at 25 °C (Fig. 1e), it can be found that a new peak appears at ~372 cm-1 at the interface. By comparing it with the characteristic peaks of the standard material, it can be determined that the reaction product is Li2S. Meanwhile, a thickened Li2S interface layer was detected at 60 °C (Fig. 1f), while only a weak Li2S signal was captured at the interface at −20 °C, which is consistent with the impedance spectroscopy results. Therefore, Li2S is the main product of the interfacial reaction between Li anode and sulfide electrolyte. Although the ionic conductivity of Li2S is much lower than that of the LPSCl electrolyte itself, it is thermodynamically stable to metallic lithium and is expected to act as a protective layer to passivate the interface.
2. Study on the nanostructure of the interface layer
High-resolution cryo-TEM was used to further analyze the nanostructure of the interfacial layer between individual lithium dendrites and the sulfide electrolyte. Figures 2a and 2b show the high-resolution TEM images of the interfacial layer formed at 25 ℃ and 60 ℃ for 48 h, respectively. For the interfacial layer formed at 25 °C, it can be determined from the lattice fringes and characteristic diffraction spots in the fast Fourier transform (FFT) image that the interfacial layer is 12 nm thick single-crystal Li2S. However, when the temperature increased to 60 °C, the nanostructure changed significantly, and there was an amorphous layer with a thickness of about 3 nm in the outer layer of Li2S. Combined with the evolution of the interface impedance at 60 ℃, it can be inferred that the amorphous layer is an intermediate state of the interface reaction. Moreover, unlike the bright diffraction spots of Li2S at room temperature, the lattice diffraction in the FFT image at 60 °C is arc-shaped, which indicates the decrease of crystallinity of the Li2S layer and the appearance of low-angle grain boundaries. Clear grain boundaries and dislocations between Li2S grains can be observed within the green dashed box, where the Li2S crystallinity further decreases.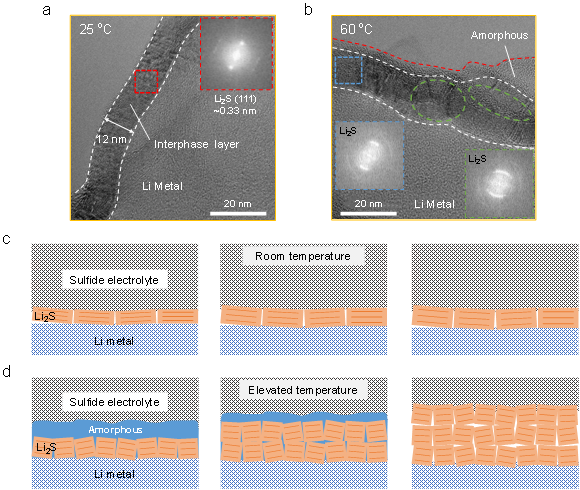
Figures 2c and 2d show schematic diagrams of the evolution of the interfacial layer nanostructures at different temperatures. At room temperature, the diffusion of metallic lithium is the controlling step of the interfacial reaction, and the reaction product is Li2S with good crystalline state. At high temperature, due to the accelerated Li diffusion and enhanced reaction kinetics, the electrolyte first becomes disordered Li-P-S-Cl atoms under Li metal reduction, and then multiple nuclei are simultaneously formed, leading to the generation of polycrystalline Li2S. The transition from disorder to order in Li2S proves that the reaction is reaction-controlled rather than diffusion-controlled in the early stage, which is consistent with the impedance spectroscopy results. As the reaction progresses, the amorphous layer continues to react with the diffused lithium, forming a significantly thicker polycrystalline Li2S layer. Therefore, unlike the well-passivated interface at room temperature, the increased thickness of grain boundaries, dislocations, and interfacial layers at high temperature leads to passivation failure, which requires additional pathways to stabilize the interface.
3. Research on the mechanism of interfacial reaction and inhibition strategy
To gain a deeper understanding of the interfacial reaction mechanism of Li-LPSCl, the researchers applied ab initio molecular dynamics simulation (AIMD) to elucidate the evolution of the interfacial structure over time from the atomic scale. Figure 3a shows the interfacial structure of Li-LPSCl after simulation at 100K and 500K. It can be found that there is higher structural disorder and reaction depth at high temperature, and the interdiffusion of S atoms in the electrolyte and Li atoms in metallic lithium is obvious. From the evolution of the radial distribution function (Fig. 3b–i), it can be found that the P–S bond is significantly broken at high temperature, accompanied by the formation of Li2S and a small amount of Li–P compounds. Simulation results indicate that weak P in sulfide electrolytes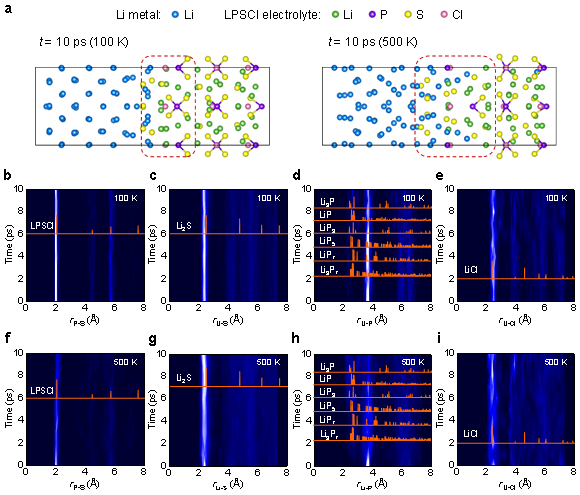
Due to the failure of interfacial passivation at high temperature, the sulfide electrolyte and Li anode cannot be in direct contact in practical applications, and artificial interfacial layer is an effective way to improve the interfacial stability. Here, we simulated six representative interfacial layer materials, including self-formed sulfide Li2S, fluoride LiF, nitride BN, oxide ZnO, and multicomponent lithium compounds Li3PO4 and Li5AlO4. The interface structure is shown in Fig. 4a–f. It can be seen that, except BN, other interface layer materials cannot resist lithium diffusion at high temperature. Combined with further Bader charge analysis (Fig. 4g), the reduction stability of the material follows the general trend of nitride > oxide > sulfide > fluoride. For the same anion, the reduction stability of multi-component compounds is lower than that of binary compounds. In contrast, nitrides have unique stability and are expected to completely suppress side reactions between lithium metal and sulfide electrolytes even at high temperatures, which are worthy of further study in the future.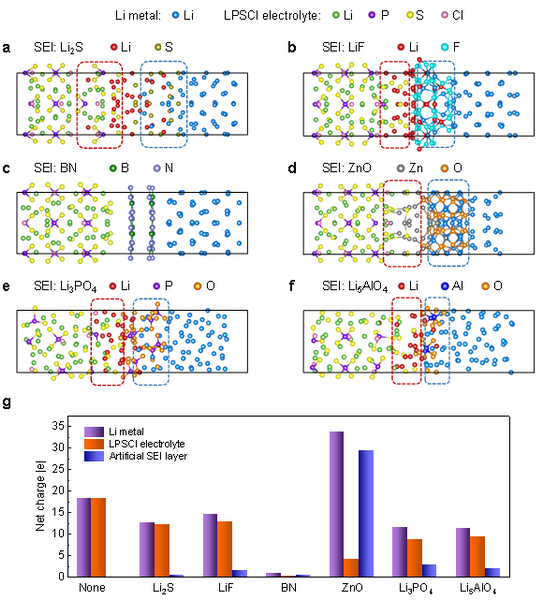
【Conclusion】
In summary, this paper systematically investigates the temperature-dependent nanostructure of the interfacial layer between a single Li dendrite and a sulfide electrolyte, and elucidates the atomic-scale interfacial structure evolution with and without artificial interfacial layers. The cryo-TEM results show that the interface layer formed at room temperature is single-crystal Li2S, which has a good passivation effect on the interface, while at 60 ℃, a large number of grain boundaries and dislocations appearing inside the interface layer lead to ultra-high interface resistance, which requires an artificial interface layer to help stabilize the interface. The simulation results show that the nitrides have ultra-high reduction stability and are expected to completely suppress side reactions. Our study provides valuable insights into the interfacial reactions of metallic lithium and sulfide electrolytes at the nanoscale and will provide rational guidance for future interface design and practical applications.

