1. Research Background
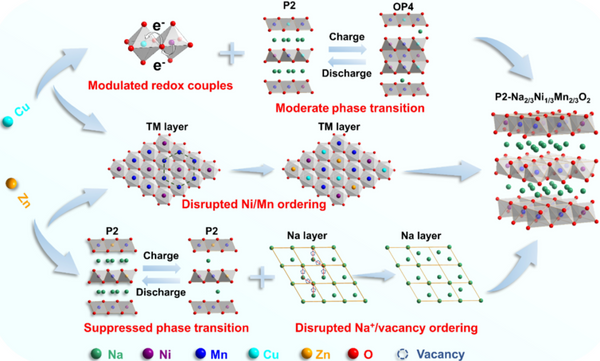
P2-type layered oxide Na2/3Ni1/3Mn2/3O2 is considered to be a promising cathode material for high specific energy sodium-ion batteries due to its high theoretical capacity and high air stability. However, its practical application faces many difficulties. : First, the bulk phase transition from P2 to O2 and irreversible oxygen release will occur when charging above 4.0 V, resulting in rapid battery capacity and voltage decay; the second is the ordered rearrangement of different sodium ions/vacancies and transition metals below 4.0 V The rearrangement reduces the sodium ion diffusion kinetics, resulting in suboptimal rate performance of electrode materials at large current densities. Although a large number of studies have shown that doping with inactive elements can effectively improve the cycle stability, it is often at the expense of capacity and voltage, and cannot fully exploit its potential high specific energy advantages; while doping with active elements can suppress irreversible oxygen release, Multiple ordered rearrangements are not affected, thereby not accelerating sodium ion diffusion kinetics. Therefore, it remains a serious challenge for Na2/3Ni1/3Mn2/3O2 cathode materials to simultaneously suppress their irreversible phase transitions, disrupt multiple ordered rearrangements, increase operating voltage, and reduce excessive oxygen release.
2. Job introduction
Recently, the research group of Prof. Zhang Liang from Soochow University proposed an optimization strategy to control the crystal structure and electronic structure of Na2/3Ni1/3Mn2/3O2 (NNMO) by co-doping with double ions to overcome this problem. Electrochemically active Cu2+ and electrochemically inert Zn2+ ions are designed to replace Ni2+ occupying the transition metal layers of Na2/3Ni1/3Mn2/3O2. Among them, Zn2+ successfully suppresses the P2-O2 phase transition and the ordered rearrangement of sodium ions/vacancies by virtue of the ionic radius similar to Ni2+ but different Fermi level characteristics; while Cu2+ not only enhances the TM-O hybrid orbital enhancement The redox potential of Ni is improved and the irreversible oxygen oxidation is suppressed. Compared with single element substitution, the synergistic effect of double ions achieves the effect of 1+1>2, and the designed P2-Na0.67Cu0.05Zn0.07Ni0.21Mn0.67O2 (NNMCZO) cathode material has both energy density and cycle stability. It has significant advantages (working voltage is 3.65 V; capacity retention rate is 91.0% for 100 cycles at 1C rate; 1000 cycles at 10C rate, only 0.48mV of voltage decay per cycle; especially at 20C high rate, it can still reaching a reversible capacity of 84.1 mAh/g).
3. Core content
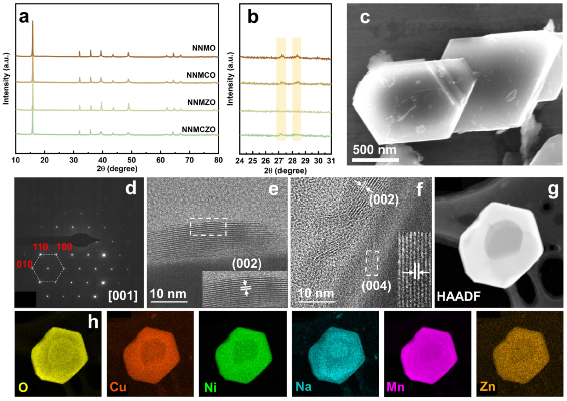
NNMO, Cu-doped Na0.67Cu0.12Ni0.21Mn0.67O2 (NNMCO), Zn-doped Na0.67Zn0.12Ni0.21Mn0.67O2 (NNMZO) and NNMCZO cathode materials were synthesized by a simple solid-phase method. As shown in Fig. 2a,b, the four materials are all P2-type layered structures. Among them, the existence of diffraction peaks at 27.2° and 28.4° indicates that the NNMO contains sodium ion/vacancy order rearrangement, and Zn doping can effectively suppress its order arrangement. Through scanning electron microscopy, transmission electron microscopy, electron diffraction and corresponding element distribution diagrams (Fig. 1c-k), it was further confirmed that the structure is a P2-type layered structure, and each element is uniformly distributed.
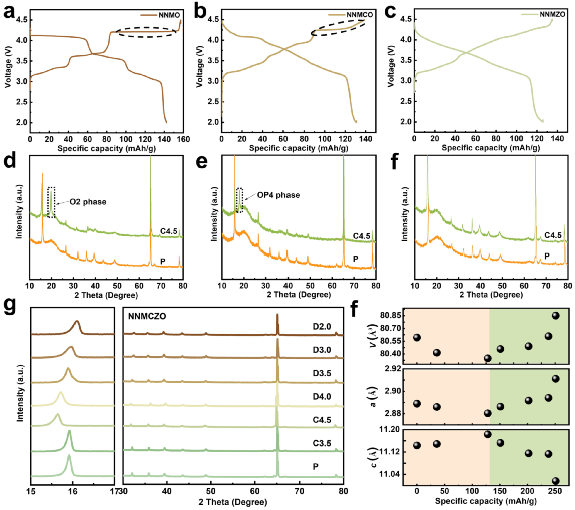
The electrochemical curves of the four layered cathode materials and their XRD patterns during charge and discharge were compared. During the charging process, NNMCO exhibits a high-voltage plateau and undergoes a moderate P2-OP4 phase transition, which is attributed to the enhanced Cu-O covalency that slows down the slip of the transition metal layer. However, there is no high voltage plateau in NMMZO, and no new phase is generated in the XRD spectrum, indicating that Zn doping effectively inhibits the phase transition. Benefiting from the Zn doping in the double ions, NNMCZO exhibits a complete solid solution reaction with only 0.63% volume strain during the entire charge-discharge process.
The valence state evolution of each metal element in NNMCZO during the first cycle of charge and discharge was studied by X-ray absorption near-edge spectroscopy (XANES). It is worth mentioning that the redox potential of nickel is increased to more than 4 V, which is higher than the reaction potential of nickel in common cathode materials. the working voltage of the cathode material. The absorption edges of Zn are completely coincident, indicating that it is an electrochemically inert element.
Using X-ray absorption spectrum expansion edge fitting analysis, it is found that the transition metal honeycomb ordered arrangement is completely broken due to the doping of double ions, so the sodium ion diffusion kinetics of the NNMCZO electrode material is improved, thus showing a very high rate performance.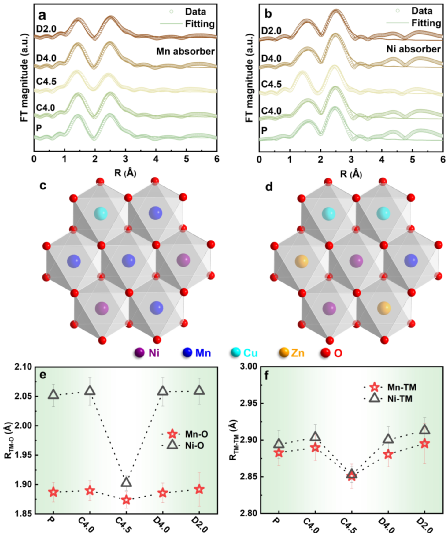
The sodium-ion half-cell was further assembled to investigate its electrochemical performance, and the related results are shown in Fig. 6. The working voltage of NNMCZO was significantly improved due to the addition of electrochemically active Cu. And due to the introduction of Zn, the phase transition is suppressed and various ordered rearrangements are broken, which shows better cycle performance and rate performance.
4. Conclusion
Overall, Zn suppresses the NNMO sodium ion/vacancy ordering and irreversible phase transition, Cu substitution improves the material's operating voltage and suppresses irreversible oxygen release, and the two-element doping compensates for the single effect and side effects brought by single-element doping. , and synergistically modulate the crystal structure and electronic structure of the cathode material, thereby greatly improving the structural stability and electrochemical performance of NNMO.

