Molecular decomposition and recombination cycles in organic aqueous flow batteries. During normal charging and discharging, so-called "zombie molecules" oscillate between DHAQ2- (left) and DHAQ4- (lower middle). When the molecule breaks down, it is converted into DHA2- (right). A voltage pulse reset breaks the molecule (right) back to its original state (left).
A new method of reversing the breakdown of key molecules could make organic aqueous flow batteries commercially viable.
While the use of renewable energy is growing, researchers continue to find the best way to store this energy so it can be used when energy sources such as solar or wind are not available.
Organic aqueous flow batteries have emerged as a promising solution, however the instability of certain materials in this technology has hindered their commercial viability.
Now, researchers at Harvard University, in collaboration with researchers at the University of Cambridge, have found a new way to solve some of the problems associated with extending the life of organic aqueous flow batteries, according to the Harvard School of Engineering and Applied Sciences ( "This is a huge step toward making these batteries competitive," said Michael Aziz, professor of materials and energy technology at the John A. Paulson School of Engineering and Applied Sciences.
Aziz and Roy Gordon, a professor of chemistry and materials at Harvard University, have been working on developing organic aqueous flow batteries using anthraquinone-like molecules for more than a decade. These molecules store and release energy and are composed of naturally abundant elements such as carbon, hydrogen, and oxygen.
While batteries made from these organic molecules are functional, the anthraquinone molecules themselves slowly break down over time, a process independent of how many times the battery is used. This breakdown limits the lifespan of batteries made from these molecules, and extending their lifespan became the team's top priority.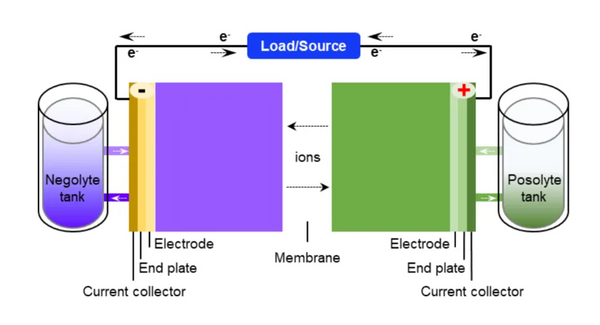
Building on previous success
The researchers have already had some success in previous work extending the life of a molecule found in the device, known as DHAQ but known in the lab as a "zombie quinone."
They found that by exposing the molecule to air at the correct time of the charge-discharge cycle, oxygen was extracted and the decomposition process reversed, returning the anthraquinone molecule to its original state. According to the researchers, it appears that the process of returning from the dead was the inspiration for the molecular moniker.
While this work held hope for the Harvard team's ultimate goal, they realized that exposing the battery electrolyte to air would be unrealistic, which meant that both ends of the battery could not be fully charged at the same time, causing inconvenience. balance phenomenon.
To find a more practical approach, Aziz and Gordon collaborated with scientists at the University of Cambridge to better understand how molecules break down. The product of the current work was born from this collaboration, an electrical method to reverse the decomposition process.
shock therapy
They say the key to the researchers' discovery of the current work is the use of "shock therapy" during the battery discharge cycle.
They found that if they performed what they called a "deep discharge," or discharge the battery's positive and negative electrodes to the point where the voltage difference was zero, and then reversed the battery's polarity so that the positive became negative and the negative became positive, they could generate Voltage pulses that reset the disintegrated molecules back to their original state.
"Usually, when running other types of batteries, you want to avoid completely draining the battery, because it degrades the battery components," Yan Jing, a postdoc at Harvard University who worked on the project, said in a press statement. This extreme discharge, which actually reverses the polarity, can reorganize these molecules, which was a pleasant surprise to us."
He explained that the process works similarly to a pacemaker, delivering a beneficial shock to the system at regular intervals, in this case reviving broken down molecules.
The researchers published the paper in the journal Nature Chemistry. In the paper, they verified that the process could produce an organic aqueous flow battery with a net lifetime 17 times longer than previous studies.
road ahead
In addition, the researchers say follow-up studies that further refine the process have shown an even greater increase in lifetime, up to 260 times as much, with a loss rate of less than 10 percent per year. According to Aziz, the latter data is particularly promising for the commercialization of the device.
"A single-digit percentage loss per year is really good for widespread commercialization, because filling up storage tanks at a rate of a few percent per year is not a major financial burden," Aziz said.
The Harvard Office of Technology Development has patented key technologies for the project and licensed this and other related patents to Quino Energy, a start-up dedicated to the commercial development of organic water-based flow batteries, Aziz, Gordon Working with their team is also in the company.

