A research team led by professors Hongyi Li and Tetsu Ichitsubo from Tohoku University, Japan, discovered that a multivalent cationic additive can limit the growth of dendrites in rechargeable batteries by changing the solvation structure of lithium or sodium ions in the electrolyte.
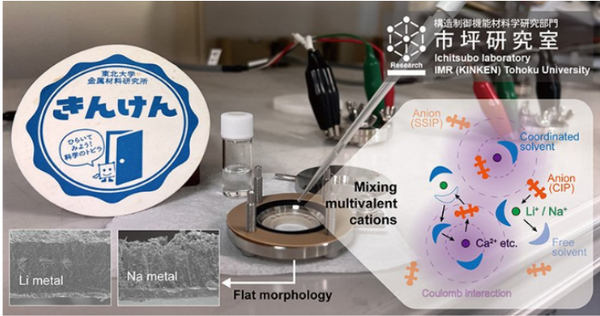
In their article published in Cell Reports Physical Science, the researchers stated: "Specifically, by focusing on CaTFSA2 as an exemplary additive, we revealed that by altering the solvation structure in dicationic electrolytes, Dendrite-free morphology during alkali metal electrodeposition."
"Our improved structure slows down the reduction of lithium or sodium ions on the electrode surface and achieves stable diffusion and electric field," said Prof. Li.
According to Tohoku University Research News, "This discovery prevents potential battery degradation and short circuits, and paves the way for higher energy density metal anode batteries."
For the next step, Li and Ichitsubo hope to improve the interface design of the metal anode to further improve the cycle life and power density of the battery.

