In order to meet the needs of EV and large-scale static energy storage markets, lithium batteries are gradually developing towards higher energy density, cheaper, safer and longer life. The energy density of lithium batteries can be improved by increasing the material specific capacity and average operating voltage.
However, the structural stability of electrode materials and parasitic reactions inside lithium batteries seriously affect the cycle life of lithium batteries. So what is the main reason?
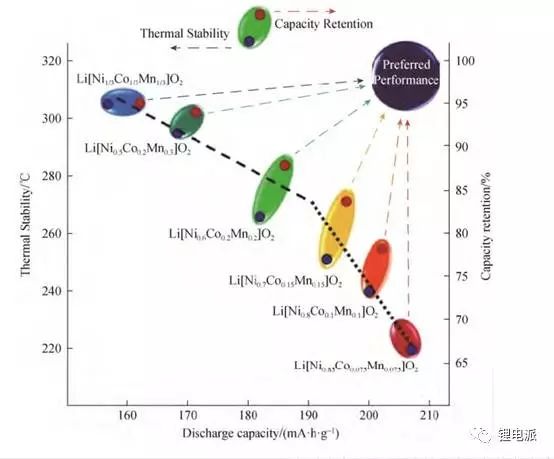
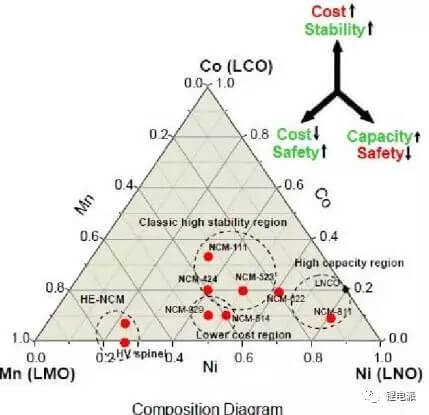
Nickel-cobalt-manganese ternary material is one of the main materials of current power batteries. Three elements have different meanings for positive electrode materials. Nickel element is to improve battery capacity. The higher the nickel content, the greater the specific capacity of the material.
The specific capacity of NCM811 can reach 200mAh/g, the discharge platform is about 3.8V, and it can be made into a battery with high energy density.
However, the problems of NCM811 battery are poor safety and rapid cycle life decay. What are the reasons that affect its cycle life and safety, and how to solve this problem? Let's analyze in depth:
The NCM811 was made into a button battery (NCM811/Li) and a soft pack battery (NCM811/graphite), and the gram capacity and the capacity of the full battery were tested respectively.
The pouch cells were divided into 4 groups for single-factor experiments, and the parameter variable was the cut-off voltage, whose values were 4.1V, 4.2V, 4.3V, and 4.4V, respectively. First, the cells were cycled 2 times at a rate of 0.05C, followed by cycling at 30°C at a rate of 0.2C. After 200 cycles, the cycle curve of the soft pack battery is shown in the figure below: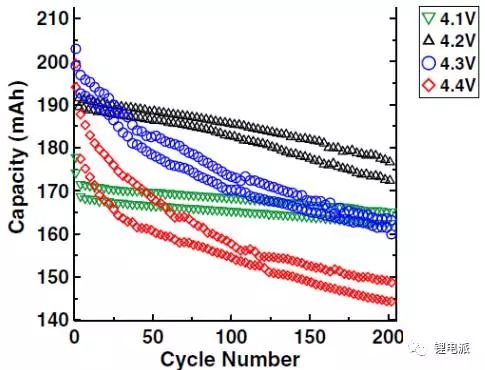

It can be seen from the figure that under the condition of higher cut-off voltage, the gram capacity of the active material and the battery capacity are both high, but the battery capacity and the gram capacity of the material decay faster. On the contrary, at a lower cut-off voltage (below 4.2V), the battery capacity decays slowly and the cycle life is longer.
In this experiment, the parasitic reactions were investigated by isothermal calorimetry and the structural and morphological degradation of the cathode material during cycling was investigated by in-situ and ex-situ XRD and SEM. conclusion as below:
1. Structural changes are not the main reason for battery cycle life degradation
The results of ex-situ XRD and SEM data show that the uncycled battery pole piece and the battery with cut-off voltages of 4.1V, 4.2V, 4.3V, and 4.4V were cycled for 200 times at a rate of 0.2C. There is no significant difference in structure.
Therefore, the rapid structural change of the active material during the charging and discharging process is not the main reason for the degradation of the battery cycle life.
On the contrary, the parasitic reaction between the interface of the highly active active material particles in the electrolyte and the delithiation state is the main reason for the shortened battery life under 4.2V high-voltage cycling.
(1)SEM
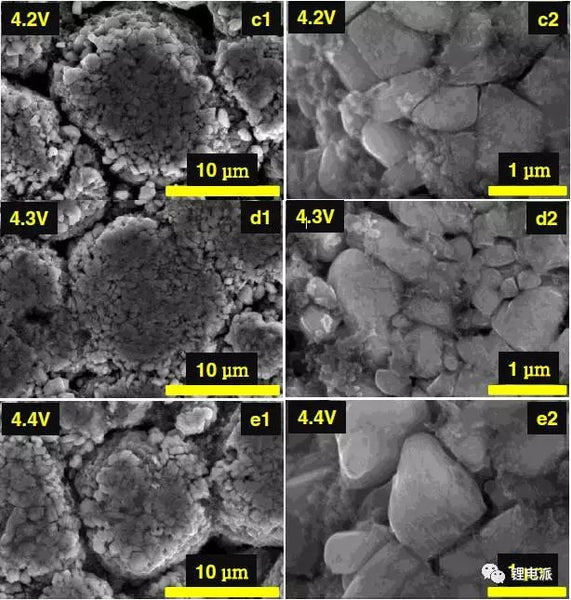
a1 and a2 are SEM pictures of uncycled batteries. b~e are the SEM images of the positive electrode active material after 200 cycles under the condition of 0.5C, the charge cut-off voltage is 4.1V/4.2V/4.3V/4.4V, and the left side is at low magnification, and the right side is at high magnification Under the microscope picture.
It can be seen from the above figure that there is no significant difference in particle morphology and degree of fragmentation between the cycled battery and the uncycled battery.
(2) XRD
It can be seen from the above figure that there is no obvious difference between the five in terms of peak shape and position.
(3) Changes in lattice parameters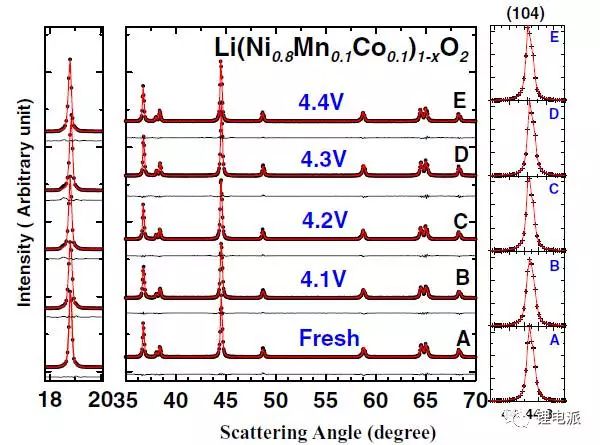
As can be seen from the table, the following points:
1. The lattice constant of the uncycled pole piece is consistent with that of the NCM811 active material powder. When the cycle cut-off voltage is 4.1V, the lattice constant is also indistinguishable from the former two, with a small increase in the c-axis. Looking at the c-axis lattice constants of the cycle cut-off voltages of 4.2V, 4.3V, and 4.4V, there is no significant difference from 4.1V (the difference is 0.004 angstroms), while the data on the a-axis is quite different.
2. There was no significant change in Ni content in the five groups of comparative experiments.
3. The pole piece with a cycle voltage of 4.1V at 44.5° exhibits a larger FWHM, and the other comparison groups are relatively close.
During the charging and discharging process of the battery, the c-axis shrinks and expands greatly. At high voltages, the decrease in battery cycle life is not due to changes in the structure of the active material. Therefore, the above three points verify that the structural change is not the main reason for the battery cycle life degradation.
2. The cycle life of NCM811 battery is related to the parasitic reaction in the battery
NCM811 and graphite are made into soft pack batteries, and the two use different electrolytes. The two groups of comparative experimental batteries were added with 2% VC and PES211 respectively, and the capacity retention rates of the batteries after cycling were quite different.
When the cut-off voltages of the batteries with 2% VC are 4.1V, 4.2V, 4.3V, and 4.4V, respectively, the capacity retention rates of the batteries after 70 cycles are 98%, 98%, 91%, and 88%, respectively. However, after only 40 cycles of the battery with PES211, the capacity retention rate dropped to 91%, 82%, 82%, and 74%.
Important: In previous experiments, the cycle life of NCM424/graphite and NCM111/graphite systems with PES211 was better than that with 2% VC. This leads to the hypothesis that in high-nickel material systems, electrolyte additives have a large impact on battery life.
The cycle life at high voltage is much worse than at low voltage. By fitting functions to polarization, ΔV and cycle number, the following figure is obtained:
When the battery is cycled at a low cut-off voltage, the △V of the battery is small, and when the voltage rises above 4.3V, the △V increases sharply, and the battery polarization increases, which greatly affects the battery life.
The rate of change of ΔV of VC and PES211 is different, which further verifies that the degree of polarization and speed of the battery are different with different electrolyte additives.
The parasitic reaction probability of the battery is analyzed by isothermal microcalorimetry, and the parameters such as polarization, entropy, and parasitic heat flow are extracted to make a functional relationship with rSOC, as shown in the following figure: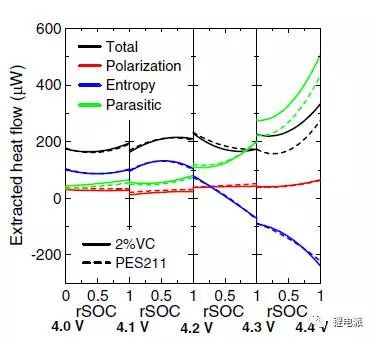
Above 4.2V, the graph shows a sudden increase in parasitic heat flow because the highly delithiated positive surface is highly reactive with the electrolyte at high voltages. This also explains why the higher the charge and discharge voltage, the faster the battery capacity retention rate drops.
3. NCM811 is less secure
Under the condition of continuously increasing ambient temperature, the activity of NCM811 reacting with electrolyte in the charged state is much greater than that of NCM111 reacting with electrolyte. Therefore, it is difficult for the battery made by NCM811 to pass the national compulsory certification.
The figure is a graph of the self-heating rate of NCM811 and NCM111 between 70°C and 350°C. The figure shows that at about 105 ℃, NCM811 began to heat up, while NCM111 did not, and it did not start to heat until 200 ℃.
Starting from 200°C, NCM811 has a heating rate of 1°C/min, while NCM111 is still 0.05°C/min, which also means that NCM811/graphite system batteries are more difficult to pass mandatory safety certification.
High nickel active material is bound to be the main material for high energy density batteries in the future. How to solve the problem of NCM811 battery life decaying too fast?
One is to improve its performance by modifying the particle surface of NCM811. The second is to use an electrolyte that can reduce the parasitic reaction between the two, thereby improving its cycle life and safety.

