The use of battery material blending to exert the synergistic effect between different materials is an effective method for the design of lithium-ion battery electrode materials. Studies have shown that the blending of two or more materials has the potential to improve the overall performance of electrodes. This strategy can not only use the synergistic effect between materials to make up for the shortcomings of the material itself, but also often produce spillover effects. Compared with individual materials, blended materials exhibit more balanced and excellent comprehensive properties.
The so-called blending refers to the physical or mechanical combination of two or more objects. Unlike the common modification methods such as hybridization and coating, blending retains the pure components of the blended materials, and the operation process is simple. It has low requirements, high composition and performance consistency, and is easy for industrial production.
Lithium battery cathode material blending modification
At present, a variety of cathode materials have been successfully developed and applied, including LiCoO2, lithium manganate, lithium iron phosphate and ternary materials. However, they also have certain shortcomings, and it is difficult to meet the requirements of ideal cathode materials. For example, LiCoO2 has poor structural stability under high pressure conditions and is expensive; lithium iron phosphate has a low redox potential and poor conductivity; lithium manganate has low mass specific capacity, etc.
① Blending of high and low safety cathode materials
Layered materials are often used as positive electrode materials for power batteries due to their high energy density, but their poor thermal stability limits their further development and application. The researchers blended layered lithium cobalt oxide and lithium iron phosphate, and proposed a unique blending method to improve the safety of layered materials. As shown in Figure 1, they demonstrated a lithium cobalt oxide and iron phosphate Lithium double-layer electrode, in which the lithium iron phosphate layer is used as both the active material and the resistive barrier layer for overcharge and thermal runaway protection. The temperature drops to 80°C, which significantly improves the safety of lithium cobalt oxide. The study found that when LiCoO2 and LiMn2O4 were blended at a mass ratio of 3:2, the cost dropped significantly, and the stable capacity was as high as 137mAh/g, and the capacity retention rate reached 92.3% after 5 cycles of overcharging. When lithium cobalt oxide and lithium iron phosphate are blended at a mass ratio of 1:1, the overcharge resistance and thermal stability are significantly improved. Under the condition of 3C/10V overcharge, it only bulges, without smoke and fire; And under short-circuit and overcharge conditions, the surface temperature of the blended material is significantly lower than that of the lithium cobalt oxide material.
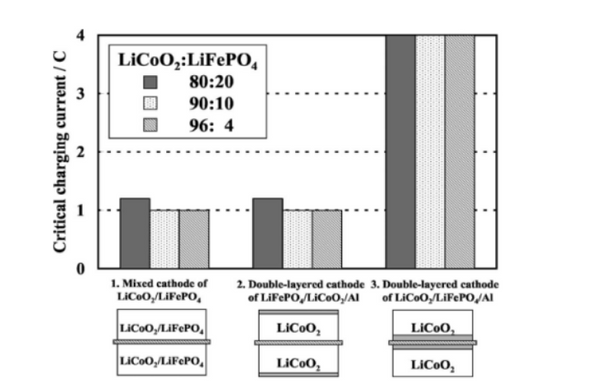
② Blending of high and low voltage cathode materials
Lithium iron phosphate has attracted the attention of researchers and field practitioners because of its high specific capacity, high safety and low cost, but because its discharge platform voltage is only about 3.6V, the energy density is reduced. The voltage of the spinel lithium manganese oxide platform is as high as 4.1V. The researchers blended lithium iron phosphate and lithium manganese oxide. The experimental results show that when the mass ratio of the two is 5:5, the average working voltage of the blended positive electrode-graphite full battery can Increased to 3.64V, compared with the pure lithium iron phosphate-graphite voltage platform, there is a significant improvement. Not only that, since lithium iron phosphate is a fine particle of submicron size, while lithium manganate is a micron-sized particle, lithium iron phosphate can be filled in the gap of lithium manganate, effectively blocking the direct contact between the electrolyte and lithium manganate, Inhibit the dissolution of manganese, but also improve the electron transport between particles, reduce the internal resistance of the electrode, improve cycle performance and stability.
③ Blending of reversible/irreversible cathode materials
With the increasing demand for high-energy-density batteries in lithium-ion batteries, the application of alloy anodes has attracted attention. However, alloyed negative electrodes generally have low coulombic efficiency (≤85%), which does not match the high coulombic efficiency of positive electrode materials. During the first charge and discharge process, some active lithium ions in the positive electrode material are lost, which reduces the utilization rate of the positive electrode and the energy density of the battery. Some researchers have coated a layer of Li2S with irreversible delithiation capacity on the surface of lithium iron phosphate cathode material. Since Li2S is charged for the first time in the positive electrode operating voltage range (2.5-4V), the delithiation capacity is as high as 1093mAh/g, while the lithium insertion capacity is only 9mAh/g when it is discharged. Therefore, after a small amount of Li2S is blended with lithium iron phosphate positive electrode, the delithiation capacity of the first charge can reach 200mAh/g. When discharging, only lithium iron phosphate plays a role, and its lithium intercalation capacity is only 156mAh/g; when the blended electrode is combined with After the Si/graphite negative electrode is matched, the extra 44mAh/g can be used to make up for the lithium ion consumption during the first charge and discharge of Si/graphite, so that the lithium ion received by the positive electrode can reach 150mAh/g, making the utilization rate of the positive electrode material close to 100%. The blended electrode not only improves the energy density of the battery, but also reduces the cost of the battery.
Blending modification of lithium battery negative electrode materials
① Blending of high and low capacity anode materials
Graphite is the mainstream lithium battery negative electrode material. Its structure is stable during charging and discharging, its volume changes little, and it has good electrical conductivity. However, due to its low theoretical capacity, it is difficult to meet the high capacity demand of the next generation of lithium-ion batteries. Silicon not only has a high capacity and is rich in resources, but its volume expansion during charging and discharging makes its cycle stability poor. Improving the cycle stability of silicon anodes is an urgent problem to be overcome, and silicon-carbon blending is an effective strategy to balance the performance of silicon anodes.
The researchers blended graphite with 5%, 10%, 15% and 20% silicon and found that the capacity increased with Si content, and the irreversible capacity also increased. When the blending ratio of Si is 20%, the reversible capacity is as high as 830mAh/g, which is twice the capacity of pure graphite, and the Coulombic efficiency in the first week is 83%, and its comprehensive performance is balanced. Some researchers have ball-milled graphite and phosphorus-doped silicon material blends, the composition of the blended material is uniform, and the excellent electrical conductivity of graphite is used to increase the power density of the material; at the same time, because graphite effectively covers the surface of silicon, the silicon material and The isolation of the electrolyte avoids the direct production of SEI on the silicon surface and improves the cycle stability of the composite electrode. As shown in Figure 2, when the ratio of graphite to doped silicon is 1:1, the blend electrode exhibits a performance of 1427mAh/g The initial capacity can still reach 883.4mAh/g after 200 cycles.
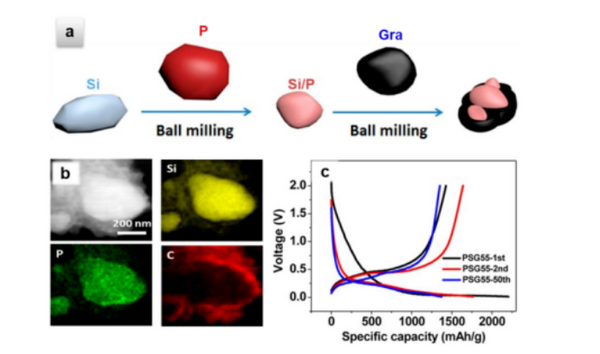
Blending modification of lithium battery negative electrode materials
② Blending of high and low Coulombic efficiency anode materials
Hard carbon materials with an amorphous structure have low Coulombic efficiency during the first charge and discharge, which limits the improvement of battery energy density. The researchers used mechanical ball milling to blend graphite and hard carbon. The graphite/pyrolytic carbon blend material has the characteristics of both graphite and pyrolytic carbon materials. As the proportion of graphite increases, the first-week Coulombic efficiency of the blended negative electrode increases. When the mass ratio of graphite and hard carbon is 2:1, its first-week Coulombic efficiency increases to 76%, which is significantly higher than that of pure hard carbon material of 69%. Coulombic efficiency, and its rate performance and cycle life are also better.
In addition, as an ideal anode material for sodium-ion batteries, hard carbon materials have excellent comprehensive performance, but due to the low carbonization temperature (less than 1000°C), the specific surface area is large, and there are many surface defects and impurity atoms, which makes it possible to consume available sodium for the first time. More ions. Soft carbon has higher structural order and fewer surface defects, which leads to higher Coulombic efficiency in the first week, but its specific capacity is lower than that of hard carbon. Some researchers have designed blended anode materials with different ratios of hard carbon and soft carbon, and used them as anodes for low-cost sodium-ion batteries. When the mass ratio of hard carbon/soft carbon is 5:2, the blended electrode exhibits the highest capacity of 282mAh/g and an initial coulombic efficiency of 80%, which is more than double the initial coulombic efficiency of 37% for pure hard carbon .

