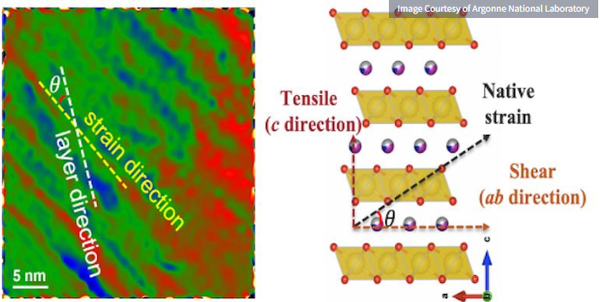
 Transition electron microscopy image of a newly synthesized cathode material for a sodium-ion battery (left).
Transition electron microscopy image of a newly synthesized cathode material for a sodium-ion battery (left).
The top graph shows the strain and stress developed in the layered cathode structure when cycled (right graph)
Energy Department researchers have made progress toward creating better, cheaper energy storage devices for commercial use.
Researchers at the U.S. Department of Energy (DoE) have made a key discovery they say could improve the performance of a battery chemistry that could potentially replace lithium-ion batteries in electric vehicles.
A research team at the Department of Energy's Argonne National Laboratory has discovered a key factor in the performance limitation of sodium-ion batteries after multiple charge-discharge cycles. The discovery paves the way for new designs for this type of battery, which could be used in electric vehicles as well as other commercial uses, the researchers said.
For several reasons, sodium-ion batteries are the best option to replace lithium-ion batteries in the future. Sodium is a naturally abundant material and has a much lower environmental impact and is less expensive than sources of lithium. In addition, the researchers say, sodium-ion batteries have a high energy storage capacity at any given weight or volume when cycled at high voltages (4.5 volts).
The team found defects in the atomic structure during the preparation of cathode materials for sodium-ion batteries, the researchers said. These defects ultimately lead to a structural earthquake-like situation in the cathode, with a catastrophic drop in performance during the cycle phase of the battery.
The researchers said that the current technology helps battery developers to adjust synthesis conditions to create superior sodium-ion cathodes for batteries, thereby improving battery performance.
"Our insights are critical for large-scale production of improved sodium-ion cathodes," Argonne Distinguished Research Fellow Khalil Amine said in a press statement. "With so many materials involved, say 1,000 kilograms, temperatures can vary widely unless taken Appropriate measures, otherwise it will lead to the formation of many defects."
Exploring the synthesis of materials
The researchers work at the Argonne Center for Nanomaterials (CNM) and the Advanced Photon Source (APS), where they synthesize the cathode material so that changes in its atomic structure can be tracked in real time.
In this process, the material's makers need to slowly heat the cathode mixture to a very high temperature in air, hold it for a certain amount of time, and then quickly bring the temperature down to room temperature.
In their analysis, the researchers found that during the rapid cooling, the surface of the cathode particles became less smooth and showed large-area strain. The data also showed that during cathode cycling, a tug-of-war effect occurred in these regions, leading to cracking of cathode particles and reduced performance.
More studies have found that battery performance degrades when the cathode is at high temperatures, or 130 degrees Fahrenheit, or when the battery is in a fast charge state (eg, 1 hour fast charge vs 10 hour charge), the researchers said.
The research team published a paper in the journal Nature Communications.
Guiliang Xu, an assistant chemist in Argonne's chemical science and engineering division, who participated in the study, said the researchers plan to combine this work with earlier research on improving anodes for sodium-ion batteries to improve the performance of future devices by 20 percent to 40%.
"In addition, it is very important that the battery will maintain this performance when cycling at high voltage for long periods of time," said Guiliang Xu. This could enable more affordable EVs with longer driving ranges and lower grid energy storage costs.

