Unexpected discoveries may lead to major discoveries in batteries, fuel cells, and hot spot conversion devices in the future.
Scientists usually design a suitable solution and perform the research by carefully selecting a research problem. But unexpected discoveries may occur in this process.
Mercouri Kanatzidis, a professor jointly hired by Northwestern University and the Argonne National Laboratory of the U.S. Department of Energy (DOE), obtained unexpected gains when he studied a new type of superconductor. This superconductor is a material that is only 4 atoms thick and can only study the movement of charged particles in a two-dimensional space. Such research may stimulate the invention of new materials for various energy conversion devices. picture
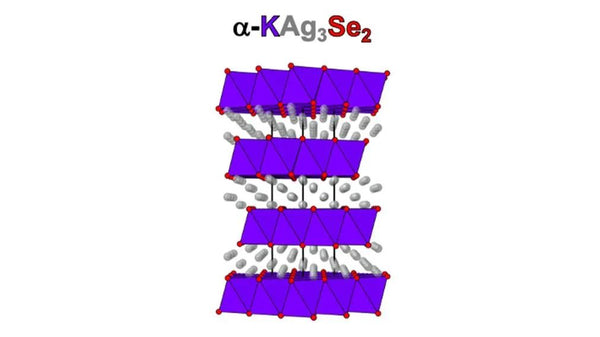
The two-dimensional superionic conductor α-KAg3Se2 has a four-layer atomic structure. The color of the atom is the same as the color on the name of the atom. (Photo courtesy of Mercouri Kanatzidis, Northwestern University and Argonne National Laboratory)
Northwestern University and Argonne National Laboratory jointly appointed Professor Mercouri Kanatzidis, said: "Our analysis shows that before the transformation, the silver ions were fixed in a two-dimensional confined space, but after the transformation, the silver ions began to move. ."
The target material of Kanatzidis is a combination of silver, potassium and selenium (α-KAg3Se2), which is a 4-layer structure. These two-dimensional materials have only length and width, but only 4 atoms high, almost no height.
When the temperature is cooled to a very low level, the superconductor material will lose all resistance to the movement of electrons. Kanatzidis, a senior scientist at the Argonne Material Science Department (MSD), said: "This is disappointing to me. This material cannot be a superconductor at all, and we have not been able to make a superconductor. But what surprised me was that this material could actually be a superconductor. Become a super ionic conductor."
In superionic conductors, the charged ions in the solid material flow freely like the liquid electrolyte in the battery. This results in solids having very high ionic conductivity, which is a measure of conductivity. It has high ionic conductivity and low thermal conductivity means that heat cannot pass through easily. This characteristic makes super ionic conductors a super material for energy storage and conversion devices.
The first clue to the research team's discovery of a material with special properties is that when the temperature is heated to between 450 and 600 degrees Fahrenheit, the material becomes a more symmetrical layered structure. The team also found that when the temperature drops and then rises to the high temperature zone again, the transformation is reversible.
Kanatzidis said: "Our analysis results show that before the transformation, silver ions are fixed in the two-dimensional space of the four materials. But after the transformation, the movement space of silver ions expands." Although people are concerned about the ions in the three-dimensional space. There is a lot of knowledge about motion, but very little is known about how they move in two-dimensional space.
Scientists have been looking for a typical material to study the movement of ions in two-dimensional materials. This layered potassium-silver-selenium material seems to be one of them. The research team measured the dispersion of ions in the solid, and found that the equivalent of this ion and heavy brine electrolyte, which is one of the fastest known ion conductors.
Although it is still too early to determine whether this superionic material with special properties can be used in practical applications, this superionic material can immediately become the key to designing other two-dimensional materials with high ionic conductivity and low thermal conductivity.
MSD principal materials scientist Duck Young Chung said: "These characteristics are very important for the design of new two-dimensional solid electrolytes for batteries and fuel cells."
Research on this kind of superionic material also helps to design new thermoelectric materials that convert the heat in power plants, industrial processes, and even automobile exhaust into electricity. This type of research can be used to design membranes for environmental purification and water desalination.
The research was published in "Nature Materials" under the title "A two-dimensional type I superionic conductor" ("A two-dimensional type I superionic conductor"). In addition to Kanatzidis and Chung, the authors include Alexander J. E. Rettie, Jingxuan Ding, Xiuquan Zhou, Michael J. Johnson, Christos D. Malliakas, Naresh C. Osti, Raymond Osborn, Olivier Delaire and Stephan Rosenkranz. The research team included researchers from the Argonne Laboratory, Northwestern University, DOE’s Oak Ridge National Laboratory, University College London and Duke University.
The research team used the Spallation Neutron Source of Oak Ridge National Laboratory and the Integrated Molecular Structure Education and Research Center of Northwestern University, and the Argonne Advanced Photon Source of the DOE Science User Facilities Department. (Argonne's Advanced Photon Source) beam line 17-BM-B. Their computer simulation uses computing resources provided on Bebop, an Argonne high-performance computing cluster.

