Today, lithium-ion batteries have been integrated into our daily life, but the content of lithium in the earth's crust is low, so it is urgent to find elements that can replace lithium to prepare high-performance secondary rechargeable batteries. As a substitute for lithium, aluminum is abundant in the earth's crust, but its chemical properties are very active, and it is easy to form a dense oxide layer, and it is also prone to aluminum dendrites as a negative electrode, resulting in short circuit of the battery, so it is difficult to realize industrialization.
Recently, in a study published in "Nature", in order to develop high-performance aluminum batteries without dendrites, researchers from Peking University, the Massachusetts Institute of Technology and other institutions jointly developed an inorganic chloride ( A low melting point molten salt electrolyte composed of sodium chloride-potassium chloride-aluminum chloride) replaces the currently commonly used ionic liquid electrolyte.
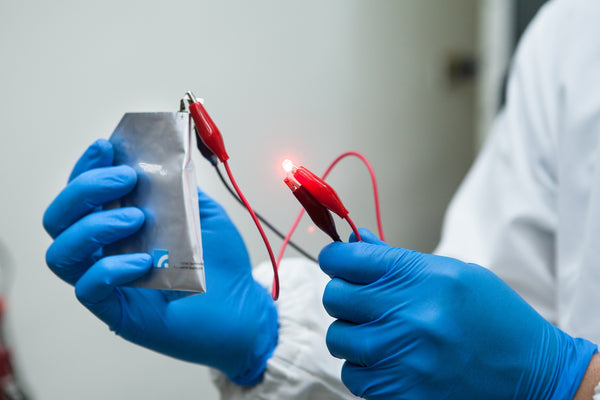
It is reported that the aluminum electrode in the sodium chloride-potassium chloride-aluminum chloride electrolyte has the characteristics of anti-dendritic growth, and the electrode surface presents a clear cut surface without sharp dendrites. This research provides new ideas for the development of future aluminum batteries.
Compared with lithium batteries, it has many advantages
The aluminum battery uses metal aluminum as the negative electrode, chloroaluminate-based molten salt or ionic liquid as the electrolyte, aluminum deposition/stripping occurs on the negative electrode, and chloroaluminate ion or aluminum ion insertion/extraction or conversion reaction occurs on the positive electrode. A battery for charge storage and release.
"Aluminum batteries have the advantages of high battery capacity, high safety and long service life." Wang Wei, a professor and doctoral supervisor of the School of Metallurgy and Ecological Engineering, University of Science and Technology Beijing, said that compared with traditional lithium batteries, aluminum batteries have many advantages , because the theoretical mass specific capacity of aluminum is 2.98 amp-hours/g, second only to lithium; the theoretical volumetric specific capacity is 8.05 amp-hours/cubic centimeter, ranking first among all metals, so the battery capacity of aluminum batteries has a very high Theoretical upper limit, and because the anode metal of aluminum batteries has stable properties and the electrolyte is not flammable, the aluminum battery will not burn, explode and other dangerous phenomena even if it is punctured and short-circuited.
In 2015, Wang Wei's research group developed a new type of non-aqueous aluminum battery with room temperature ionic liquid as the electrolyte, graphite as the positive electrode, and aluminum as the negative electrode. This battery system has a discharge voltage of up to 2 V, and has excellent cycle stability and rate performance, showing great practical potential. Since then, non-aqueous aluminum batteries have gradually become a research hotspot in the field of energy storage worldwide.
However, in the follow-up research process, relevant researchers found that due to the extremely strong acidity of ionic liquid electrolytes, high-capacity cathode materials dissolved in electrolytes usually have the problem of short cycle life. Water absorption, poor air stability, and easy decomposition, so the development of electrolytes has become an important research direction for non-aqueous aluminum batteries.
"This latest research, by using a low-melting inorganic chloride molten salt electrolyte, successfully replaces the currently commonly used ionic liquid electrolyte, and achieves high-rate operation, low-voltage polarization and high energy efficiency of aluminum batteries." Wang Wei said that due to the high thermal stability and non-flammability of the low-melting-point molten salt electrolyte, the safety problem of large-scale integrated systems is solved. "From the development of room temperature ionic liquid electrolytes to the construction of a series of high-voltage, high-capacity aluminum battery systems, a large number of research results have promoted the practical application of non-aqueous aluminum batteries," he said.
Aluminum batteries still have "fly in the ointment"
Although aluminum batteries have good application prospects and have made significant breakthroughs in recent years, their shortcomings, such as insufficient reaction kinetics, low energy density, and serious capacity decay in some systems, still need to be improved.
It is reported that in the aluminum battery system using ionic liquid electrolyte, the graphite material based on the intercalation/deintercalation reaction mechanism has limited reversible capacity, which will lead to a low overall energy density of the battery; cathode materials such as sulfur based on the conversion reaction mechanism, Operating at a lower temperature, it exhibits the characteristics of high specific capacity, but it has the disadvantages of slow battery reaction kinetics, large charge-discharge voltage polarization, poor charge-discharge rate performance, and short cycle life, which will greatly reduce the battery. energy efficiency.
"Non-aqueous rechargeable aluminum-ion battery cathode materials usually face the problems of low conductivity and structural disintegration." Wang Wei introduced that the volume change of the embedded material during the cycle process, in addition to the deterioration of the material's conductivity, It will also lead to swelling and disintegration of electrodes, pulverization of active materials, etc. In addition, intercalated transition metal compounds still have the problems of low discharge voltage, low capacity, and rapid capacity decay, which are much more serious than Li-ion batteries. Conversion materials generally face the problems of irreversible reaction and low coulombic efficiency (the percentage of charge released during battery discharge to the number of charges input during charging under given conditions), and the discharge capacity of the battery is low in the first few cycles. After a sharp decline, it exhibits rapid capacity fading and poor cycling stability.
At the same time, aluminum as a negative electrode material has a passivation layer on the electrode surface, which will reduce the voltage and efficiency of the battery; severe corrosion of aluminum will also lead to irreversible aluminum consumption, thereby reducing the utilization rate of aluminum electrodes; and during the cycle process. The growth of aluminum dendrites will also reduce the safety and cycle life of the battery.
According to Wang Wei, some of the current obstacles to the commercialization of non-aqueous rechargeable aluminum-ion batteries also include the lack of cheap, corrosion-resistant current collectors and non-decomposable binders that can operate stably in acidic AlCl3-based electrolytes. "At present, except for expensive materials such as glassy carbon, tantalum, platinum, etc., there are few stable and cheap materials that can be used as current collectors." He said that in non-aqueous rechargeable aluminum-ion batteries, the role of binders has been neglected. , so there are fewer studies on improving battery performance through the modification of existing binder systems and the development of new binders.
Aluminum batteries have practical application prospects
It is understood that energy storage technology is closely connected with new energy applications and the development of power grids, which can effectively improve energy utilization efficiency and solve problems such as power supply in remote areas. Therefore, energy storage technology is a key link that cannot be avoided in the development of new energy. Industry insiders believe that the future of energy storage batteries should be in the wind power and optoelectronic industries, especially the wind power industry that has already been deployed in large numbers. Although the wind power industry has developed rapidly in recent years, it has been plagued by grid integration due to the instability of wind resources.
The advantages of aluminum batteries are high safety, good stability, and excellent wide temperature performance. Therefore, Wang Wei believes that aluminum batteries will be inseparable from energy storage systems, special equipment and other industries in the future.
"Today's energy storage device market still maintains a rapid development rate." Wang Wei said that by 2025, China's electrochemical energy storage market power scale will reach 28.6 GW, and the market share will reach 128.7 billion yuan. The market size has a trillion-level market potential.
Due to the characteristics of short construction period, low operating cost, and no impact on the environment, electrochemical energy storage technology has become the first choice for grid application of energy storage technology to solve new energy access. Currently, lithium-ion batteries dominate the electrochemical energy storage technology due to their high energy density. However, high cost, limited lithium resources, and safety issues greatly limit its large-scale energy storage applications.
Due to the low cost of aluminum anode, high content of crustal elements, and high specific capacity, aluminum batteries are considered to be a promising battery for practical applications other than lithium-ion batteries. More importantly, the aluminum battery system has high safety, and the new aluminum battery will solve the problem of large-scale integrated system safety after it is put into production.
Wang Wei said that in the future under the background of "double carbon", energy storage battery standards should pay more attention to the improvement of environmental, energy and resource benefits, build a standard framework for green and low-carbon circular development, and follow the principle of establishing first and then breaking, and actively and orderly advancing. . At the same time, attach importance to international cooperation, do a good job in coordination of rules, fully consider the national conditions and development stages of different countries, maximize mutual recognition of accounting rules and accounting systems, and jointly promote green and low-carbon development.

