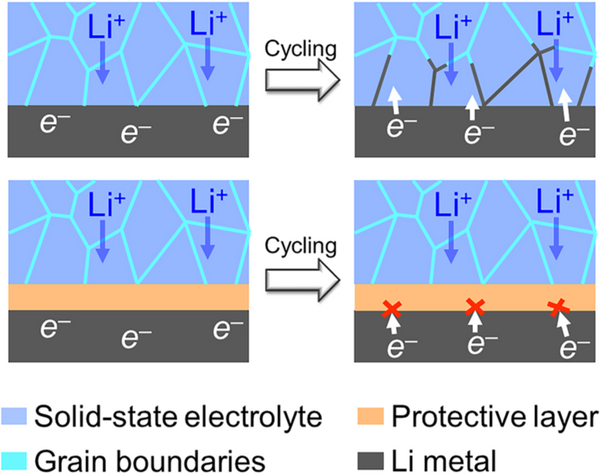
Rechargeable solid-state lithium metal batteries are considered to be the most potential next-generation energy storage battery systems due to their high theoretical specific capacity and non-flammable properties. In solid-state lithium metal batteries, the most important component is the solid-state electrolyte. The most promising materials include Li7La3Zr2O12 (LLZO), Li3PS4 (LPS) and Li1+xAlxTi2–x(PO4)3 (LATP). However, until now, solid-state lithium metal batteries have been difficult to achieve practical application, and one of the key problems is that lithium dendrites can grow and spread along the grain boundaries of solid-state electrolytes after exceeding the critical current density. The critical current is even lower than the critical current density corresponding to the organic electrolyte in conventional lithium batteries. This dendrite growth phenomenon, as well as side reactions at the interface between the solid electrolyte and the negative electrode, will lead to rapid battery decay and even failure.
Introducing a protective layer at the electrolyte-anode interface is a feasible solution to the above problems, which has gained extensive academic attention in recent years. Materials such as LiF, LiI, ZnO, and h-BN were found in previous studies to stabilize the interface between the solid electrolyte and anode, thereby suppressing Li dendrite growth and interfacial side reactions. Although the research on the interface protective layer has made remarkable progress, the development of the protective layer for solid-state lithium metal batteries is still in the preliminary stage. How to establish a systematic material screening process and quickly search for potential protective layer materials is still a challenge It is an urgent problem to be solved.
Aiming at the electrolyte-anode interface protective layer in solid-state lithium metal batteries, Pan Feng's group from the School of Advanced Materials, Shenzhen Graduate School, Peking University successfully searched out a number of inorganic materials from 2316 inorganic materials through high-throughput computational screening based on density functional theory. Potential protective layer material. According to the thermal stability, electrochemical stability, interfacial chemical reaction stability, band gap and conduction band bottom of the material, 5 kinds of protective layer materials for LLZO, 28 kinds for LPS and 7 kinds for LTP were screened out. The calculation results show that the protective layer can effectively block the electrons of the metal lithium anode from entering the solid electrolyte, thereby avoiding the reduction of lithium ions at the grain boundaries and inhibiting the growth of lithium dendrites in the electrolyte. This research greatly narrows the search space for electrolyte-anode interface protective layer materials, which is expected to accelerate the development and application of solid-state lithium metal batteries. The related results were published in the international journal Nano Energy under the title of "High-throughput screening of protective layers to stabilize the electrolyte-anode interface in solid-state Li-metal batteries".
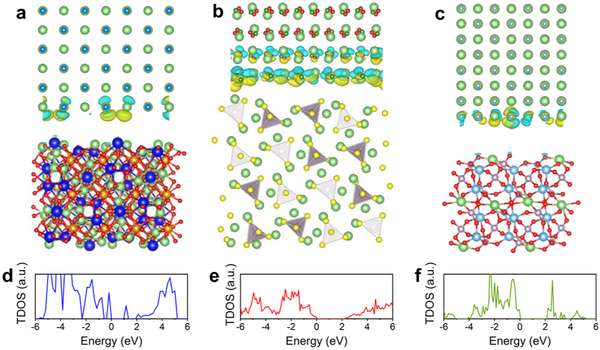
The introduction of a protective layer between the solid electrolyte and the lithium metal negative electrode of a solid-state lithium metal battery requires not only high thermodynamic and electrochemical stability, but also certain conditions in the electronic structure to inhibit the formation of lithium dendrites in the electrolyte material crystals. growth in the world. For an ideal solid electrolyte material, its conduction band bottom needs to be higher than the potential energy corresponding to the redox potential of Li+/Li0, but for three representative solid electrolyte materials, LLZO, LPS and LATP, the conduction band bottom at the surface/interface will be higher than the potential energy corresponding to the redox potential of Li+/Li0. Lower than Li+/Li0 potential energy. This results in the transfer of electrons from the lithium anode to the electrolyte grain boundaries during battery charging, where the lithium ions are reduced to lithium metal, forming dendrites. At the same time, the interfacial side reactions caused by the charge transfer further affect the performance of the battery. Therefore, in order to prevent electrons from entering the solid electrolyte, the conduction band bottom of the protective layer material needs to be higher than the potential energy of Li+/Li0, and has a higher band gap, thus hindering the electron transfer.

The research team first conducted high-throughput screening for material stability. The thermal stability screening condition is no more than 5 meV/atom higher than the ground state energy, and about 40% of the material can be screened out by this condition. The screening conditions for electrochemical stability were set for the electrochemical stability windows of LLZO, LPS and LTP, respectively. Since the phase transition of the material is governed by kinetics, a certain overpotential is required for the phase structure transition that occurs during lithium extraction/intercalation. Based on this, the electrochemical stability screening condition of the protective layer material is set such that its electrochemical stability window covers more than 90% of the protected solid electrolyte, and this step will screen out more than half of the candidate materials. The interfacial chemical reaction stability is also set for LLZO, LPS and LTP, respectively, and the absolute value of the reaction energy is less than 100 meV/atom, so the selected candidate materials are difficult to chemically react with solid electrolytes.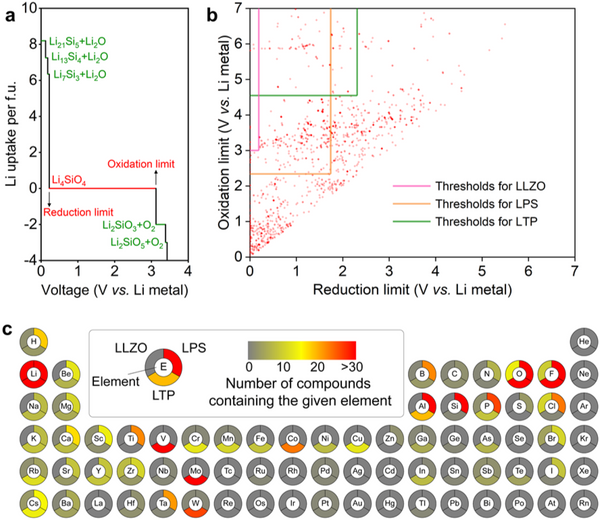
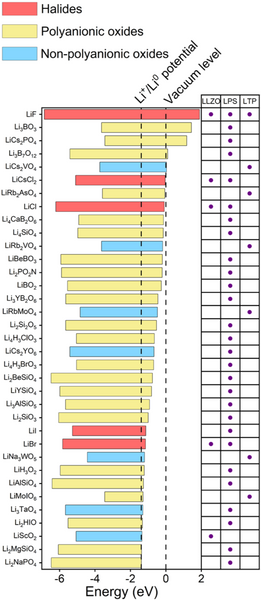
The research team then screened the material's electronic structure. The screening conditions are set as: (1) the band gap is greater than 2 eV; (2) the bottom of the conduction band is higher than the potential energy of Li+/Li0. Through this round of screening, 5, 28 and 7 kinds of protective layer materials for LLZO, LPS and LTP were finally obtained. Among them, LiF, LiI and Li2SiO3 have been reported to be beneficial to stabilize electrolytes of solid-state lithium metal batteries- negative interface, thus proving the reliability of the above screening process. Most of the screened protective layer materials are halides and polyanionic oxides, which are mainly due to the obvious ionic bond characteristics of these materials, so the materials have higher conduction band energy.
In addition, by studying the electronic structures of LLZO(101)|LiCl(001), LPS(210)|LiBO2(001) and LTP(012)|LiF(001) interfaces, it is further confirmed that the band gap will be maintained at the interface, thereby avoiding Transfer of charge from the protective layer to the electrolyte. The electronic structure calculation of the LiCl(001)|Li(110) interface verifies that under the above band structure screening, electrons cannot enter the conduction band of the protective layer material from the lithium metal anode, thus avoiding the possibility of entering the solid electrolyte. These results suggest that the candidate protective layer materials can effectively hinder the electron transfer at the electrolyte-anode interface, thereby suppressing the Li dendrite growth and the occurrence of side reactions.