Lithium-ion batteries have been widely used in many electronic products, new energy vehicles, and large-scale renewable energy storage devices. In recent years, "range anxiety" has stimulated the continuous development of high specific energy lithium batteries, and the continuously optimized electrode materials and electrode processing technologies have significantly improved the energy density of batteries. However, in practical battery systems, the loss of active lithium inevitably occurs during the initial charge-discharge cycle of the negative electrode, making it difficult for almost all used electrode materials to reach their theoretical energy density values. Pre-lithiation is an important technique to improve the first cycle coulombic efficiency (ICE) of lithium-ion batteries and to meet the energy density required for practical applications. Numerous studies have focused on how pre-lithiation technology can improve the ICE of full cells, however, little attention has been paid to the effect of pre-lithiation on battery cycling stability.
【Work introduction】
Recently, researcher Cui Guanglei and Dong Shanmu from the Qingdao Institute of Bioenergy and Processes, Chinese Academy of Sciences, and Professor Wang Chunsheng from the University of Maryland, USA, published a review of "Mechanistic insight into the impact of pre-lithiation on the cycling stability of lithium-ion battery". The published article, combined with the recent findings of the research group, establishes a correlation between cycling stability and prelithiation of full cells. The article was published in the top international journal Materials Today. Sun Jinran and Huang Lang are the co-first authors of this article.
【Description of content】
In practical applications, prelithiation is the key to improve the energy density of Li-ion batteries. Pre-lithiation of high specific capacity anodes achieves an increase in initial Coulombic efficiency (ICE) by compensating for lithium loss upon solid electrolyte interphase (SEI) formation. The fact that prelithiation implemented in a full cell deepens the lithiation of the negative electrode (even if it only compensates for the loss of active lithium during the initial cycle), which in turn has some impact on the cell's cycling stability, is seldom seen followed. This paper first discusses the effect of prelithiation on the cycling stability of the full cell, which is closely related to the type of anode material, the degree of prelithiation, and the stability of the SEI. On this basis, this review proposes how to simultaneously achieve high energy density and long cycle life through prelithiation.
The effects of prelithiation on cycling stability can be classified into three cases: (1) positive effects on anodes with small volume changes, such as graphite and hard carbon materials; (2) unpredictable cycling stability of alloy/conversion anodes (3) Complex effects of composite materials such as SiO/Si/graphite. Each part discusses the corresponding mechanisms supporting cyclical changes with typical examples.
(1) The main reason for the loss of active lithium in carbon-based anode materials (such as graphite and hard carbon) is the formation of SEI (solid electrolyte interlayer) during the initial charge-discharge cycle. The strong combination of SEI and carbon can accommodate the <20% volume change of the carbon anode during lithiation/etching, and achieve long cycle life. Due to the less stress generated inside the material after pre-lithiation, the pre-lithiation anode and SEI can well maintain the structural integrity without additional lithium loss. When low-loading prelithiation was performed on this anode, the preloaded lithium was only used to compensate for the loss of active lithium associated with the initial formation of SEI in the first cycle. The full-cell cycle rate after pre-lithiation was almost unchanged compared to that without pre-lithiation (Figure 1). For the negative electrode with a high degree of prelithiation, after compensating for the loss of active lithium in the first cycle, the preloaded lithium can exist in the negative electrode as a lithium inventory, and the loss of active lithium is continuously replenished during the cycle until it is exhausted. Therefore, pre-lithium would improve the cycling stability of the full cell.
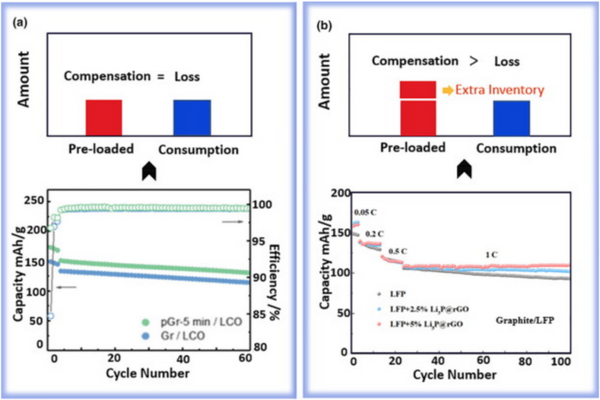
Pre-Lithium Recommendations:
Reasonably increase the amount of preloaded lithium. After compensating for the initial capacity loss caused by SEI, the preloaded lithium amount can be used as a lithium inventory to supplement the active lithium loss during long-term cycling. Therefore, the battery cycle stability can be improved. However, if prelithiation is excessive, lithium dendrites/lithium plating will be formed, resulting in more serious loss of active lithium and potential safety hazards. The recommended upper limit of preloaded lithium is as follows:
picture
Ahalf is the first anode lithiation capacity obtained in the anode-lithium metal half-cell, and Cfull is the initial lithiation (discharge) capacity obtained in the actual full cell.
(2) Alloy and conversion negative electrode
Alloy and conversion-type anodes suffer from huge volume deformation during lithiation and delithiation. Taking Si as an example, the volume change caused by lithiation of Si is drastic and nonlinear. During the initial process, lithium ions are inserted into the interstitial sites of Si to form amorphous LixSi. The electrodes undergo elastic and plastic volume expansion, which can be partially regulated by the electrode porosity. After further lithiation, a second phase reaction occurs. The sudden appearance of Li15Si4 leads to the rearrangement of the Si structure. At the same time, the volume expands dramatically. The rapidly increasing internal stress greatly threatens the structural stability of the SEI, possibly leading to irreversible loss of electrolyte and lithiated materials. Furthermore, it has been reported that for Si, the volume change rate for delithiation is faster than that for lithiation. In this process, the rapid volume shrinkage easily leads to the fracture of Si particles, which makes many Si nanoparticles lose electrical contact, and the final capacity decreases significantly.
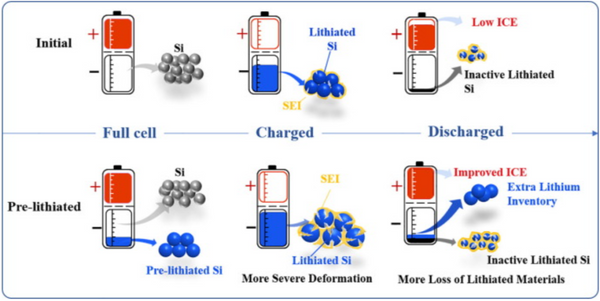
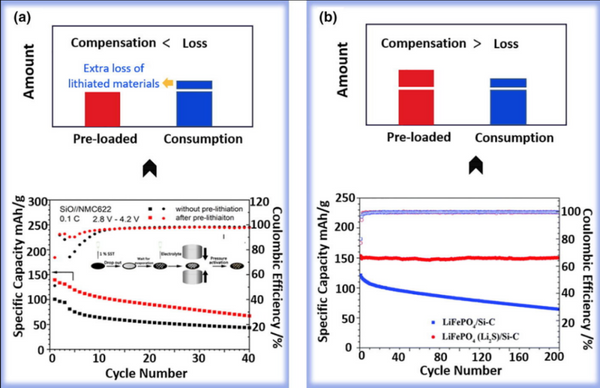
Pre-lithiation improves the ICE of the full cell, while inevitably intensifying the degree of lithiation inside the lithiated anode, which means more Li15Si4 is formed (Fig. 2). The increased Li15Si4 content seriously challenges the structural stability of the SEI as well as the subsequent cycling stability, especially in Si anodes with particle diameters over 150 nm. Among them, the strain energy stored by the electrochemical reaction and the relatively non-uniform lithiation will form a large stress on the particle surface. If the SEI fracture toughness cannot withstand the stress caused by the increase in Li15Si4, the lithium loss due to SEI reconstruction and contact failure of the lithiated material will increase. In subsequent cycles, the pre-lithiated anode was more deeply lithiated than the non-pre-lithiated anode due to the loss of active material in the first cycle, resulting in worse cycle performance (see Figure 3). As shown in Figure 3a, the cycling stability of the prelithiated SiOx/NCA full cell deteriorated significantly, and the capacity retention rate dropped from 76.97% to 61% after 100 cycles. The same situation also occurs in other systems, such as pre-lithiated SiO/NMC622 full cells. It should be noted that most of the above-mentioned pre-lithium cells do not maximize the initial discharge capacity, which means that pre-lithium only compensates for the initial cycle Lithium loss at the time. There is no additional lithium inventory in these anodes. Even if the lithium inventory is increased by increasing the preloaded lithium, the competition between lithium loss (due to deeper lithiation) and lithium compensation (additional lithium inventory) can make cycling stability (after prelithiation) impossible Predicted, as lithium inventories generate more active lithium losses at the same time.
Pre-Lithium Recommendations:
a) Properly adjust the N/P ratio and the degree of lithiation. When using a Si anode, the smaller the N/P ratio, the deeper the lithiation of the anode and the more Li15Si4 formed after pre-lithiation. A higher N/P ratio can reduce the lithiation degree of the negative electrode, and an appropriate increase in the preloaded lithium amount will form an excess lithium inventory in the negative electrode. From the relationship between the volume change curve and the specific capacity, it can be seen that when the specific capacity is 2000 mAh/g, controlling the lithiation state of the silicon negative electrode can significantly reduce the volume change compared with the fully lithiated silicon, which can greatly reduce the lithium Inventory depletion rate to improve cycle stability.
b) Optimize the composition and structure of SEI. The optimization of SEI requires a comprehensive consideration of the factors involved at the interface, namely silicon particles, binders and electrolytes. Among them, the design of the electrolyte is the key to achieve good SEI and enhance fracture toughness. Several enhanced SEIs based on smart electrolyte designs, such as the elastic polyether-enriched SEI reported by Nanda et al. and the LiF-based SEI by Chen et al., can maintain the structural integrity during cycling and effectively improve the cycling stability of Si. . The improved structural stability of SEI can protect Si from severe fracture and avoid the problems caused by the loss of active lithium.
(3) Composite negative electrode
Silicon (SiOx)-graphite is one of the most widely used composite anode materials in modern industry, and prelithiation is critical to achieve higher energy densities for these materials. In addition to the volume change caused by deep lithiation, the distribution of lithium in the lithiated anode also has a significant effect on the cycling stability. In this composite system, the competition between lithium compensation and depletion after pre-lithiation is more complicated because the lithiation potential of graphite is close to the formation potential of Li15Si4 (about 90–100 mV). Recently, by solid-state NMR, we tracked the evolution of the active lithium distribution in the lithiated SiOx-graphite composite anode before and after prelithiation (removed from a fully charged cell). The proportions of LiCx and Li15Si4 in the pre-lithiated anode were simultaneously significantly increased compared to the non-pre-lithiated anode. Due to the good reversibility of graphite, the additional storage of lithium in the increased LiCx is beneficial to prolong the cycle life. However, as mentioned earlier, Li15Si4 is the main source of electrode deformation, leading to SEI fracture and active material contact failure. The increase of Li15Si4 is extremely detrimental to the overall cycling performance in the case of insufficient graphite utilization caused by the close formation potential. Therefore, based on this complex effect, pre-lithiation makes the cycling stability of the full battery of the composite anode show different trends.
Pre-Lithium Recommendations:
a) Adjustment of component proportions. The specific capacity of the negative electrode should be increased, and the N/P ratio should be increased at the same time. With the increase of N/P ratio, appropriately increasing the proportion of silicon or SiO can reduce the degree of lithiation of silicon, thereby reducing the volume deformation of silicon. Therefore, an effective improvement in cycling stability can be achieved due to the reduction of active lithium loss and the contribution of increased lithium inventory.
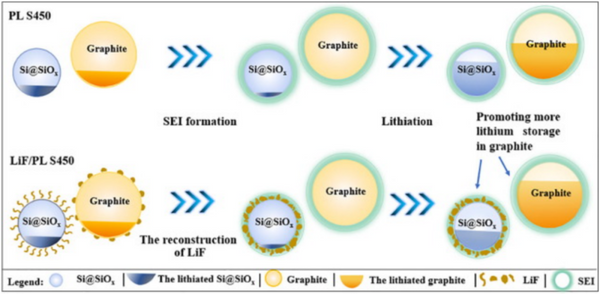
b) Development of a method to efficiently separate the lithiation potentials of LiCx and Li15Si4. Improving the utilization rate of highly reversible graphite and reducing the amount of Li15Si4 is of great significance for optimizing the cycle performance of the composite anode. For example, the LiF-induced selective lithiation strategy proposed by Sun et al. to separate the formation potentials of LiCx and Li15Si4 (Fig. 4). Due to the difference in the interfacial separation energy (Wsep, ideal work of separation, which models the energy required for an interface to ideally separate into two surfaces in a vacuum) between LiF and active materials (Si, SiO, and graphite), LiF can preferentially In situ aggregation on localized Si@SiOx particles. LiF with poor ionic and electronic conductivity slows down the lithiation kinetics of Si@SiOx particles, enabling the formation of Li15Si4 at a lower potential, which significantly improves the utilization of graphite and reduces the amount of Li15Si4. The results show that the cycling stability of the prelithiated Si@SiOx/C composite anode can be significantly improved by liberating the highly reversible lithium storage capability of graphite from the redundant capacity. In the full-cell demonstration of ultra-high areal capacity (NCA/S450, 4.9 mAh/cm2), the capacity retention can be significantly improved from 85% to 94.2% after 300 cycles.
【Conclusion and Outlook】
(1) Attention should be paid to the by-products or residues generated by the pre-lithium reagent, which may have a certain impact on the cycle stability by changing the composition or structure of the SEI, and may also interfere with the internal environment of the battery and affect the battery energy density.
(2) Attention should be paid to the safety hazards of ex-situ pre-lithiation, such as heat release during pre-lithiation, environmental stability of materials (lithium foil, lithium powder), etc.
(3) Encourage the development of multi-functional pre-lithium technologies, such as pre-lithium reagent decomposition products that can be flame retardant, pre-lithium reagents to optimize SEI, and pre-lithium one-step conversion to high-quality SEI, etc.
In summary, prelithiation is a widely used technique to improve the energy density of Li-ion batteries by supplying additional Li to compensate for Li losses. It is worth pointing out that the influence of pre-lithiation on the cycle stability of lithium-ion batteries is complex, and is closely related to the specific system, the degree of pre-lithiation and the stability of SEI. On the basis of systematically summarizing the existing literature, this paper clarifies the mechanism of cyclability change after pre-lithiation, which is of great significance to guide the application of pre-lithium technology in practical industrialization.

