What is a lead acid battery?
Lead-acid battery (VRLA) is a storage battery whose electrodes are mainly made of lead and its oxides, and the electrolyte is sulfuric acid solution. In the discharge state of a lead-acid battery, the main component of the positive electrode is lead dioxide, and the main component of the negative electrode is lead; in the charged state, the main component of the positive and negative electrodes are lead sulfate.
The nominal voltage of a single-cell lead-acid battery is 2.0V, which can be discharged to 1.5V and can be charged to 2.4V; in applications, 6 single-cell lead-acid batteries are often connected in series to form a lead-acid battery with a nominal 12V , There are 24V, 36V, 48V and so on.
Plates of spongy lead (pole -) and lead dioxide (pole +) are bathed in dilute sulfuric acid (density 1.24 to 1.30) with demineralized water. A charged battery is made up of these plates and discharged sulfuric acid, these same plates, part of which is transformed into lead sulphate in its amorphous form and low density sulfuric acid, almost water. The charge converts the amorphous lead sulphate back to sulfuric acid and reconstitutes the plates. At the end of charging, there may be gassing, oxygen and hydrogen, by electrolysis of water. So lower electrolyte level.
Amorphous lead sulphate will crystallize if given time, it is embedded on and in the plates of the batteries to form islands. Chronic underloads are a major contributor. Once lead sulphate has crystallized, it no longer converts back to sulfuric acid at the time of charging, it is too stable, and a battery charger is ineffective, the battery is said to be “sulphated”. This can happen very quickly with a starter battery left discharged, 36 hours are enough for the amorphous lead sulphate to crystallize, or less quickly from five to ten years with a well treated battery.
The second factor in degrading battery capacity is the oxidation of lead plates, the age of the battery this time being one of the main causes as well as a lack of chronic electrolyte. Even for a well-treated battery, the 15 to 20 year mark is difficult to pass, except in special cases. Regeneration will not work on an oxidized battery.
Regeneration consists of sending high-power, controlled electrical pulses. Little by little, they will break the crystalline network of lead sulphate, where an ordinary charger is ineffective. The lead sulphate will again electrolyze into sulfuric acid and reconstitute the plates. The process will last from 24 hours for a car battery, to five to ten days for large industrial batteries of several tonnes. The battery regains its capacity as a young girl. It is said to be desulfated.
Regeneration is economical, ecological and clean
Invented by the French Gaston Planté in 1859, they have conquered the world ever since. 99% in tonnage of the batteries produced in the world are lead batteries. We all have at least one in our diesel or gasoline powered vehicle. The industry consumes a lot of it in its forklifts, to overcome power outages by inverters, to store solar energy and many other applications. Almost all of the lead used to make them comes from the recycling of old batteries, lead is a toxic metal, never get rid of a lead battery by throwing it in the wild.
The nominal voltage of a lead-acid battery is 2 V (2.15 V for a fully charged battery). To obtain higher voltages, the 2 V elements are assembled in series (at the tail leu leu). Hence the name of battery, we should use the term battery of electric accumulators, but it is definitely the word of battery which was essential.
New technologies are emerging but are not ready to dethrone the old lead-acid battery which will remain queen for a long time in its field. Lithium batteries are five times more expensive and lithium is very scarce on our planet and very toxic. Cadmium nickel batteries are almost prohibited due to the toxicity of cadmium. NiMh batteries have a detestable memory effect that does not exist on the lead battery and a low number of charge-discharge cycles. Other technology will undoubtedly see the light of day, such as the lithium battery suffers and especially the Sodium-ion.
Two figures characterize a lead-acid battery: its voltage in Volts (V) is twice the number of 2 V cells making up the battery, and its capacity in Ampere hours (Ah). This capacity varies depending on the amount of electricity requested from the landfill. The discharge time will be preceded by the letter C, C10 means for a discharge in 10 hours. Traditionally the capacity of a starter battery is given in C20, traction in C5 and solar in C100. As an example, a 1000 Ah battery in C100 will only make around 800 Ah in C10, 660 Ah in C5 and will rise to 1100Ah in C240.
A third digit preceded by CCA, specific to starter batteries given in A (Ampere). This is the maximum intensity that the battery can charge for a certain number of seconds, measured according to different standards, EN (European) SAE (American) and others less common here.
Three categories of batteries.
Starter batteries. They are capable of delivering a large current intensity in a very short time. The lead plates which compose them are thin and numerous. They cannot stand to be discharged by more than 20% without reducing their lifespan and must be recharged as soon as possible after discharge. We find them in vehicles with thermal engines. Nominal density of the electrolyte 1.28 to 1.30.
Slow discharge batteries (also called traction batteries). They are built to withstand discharges up to 80% before recharging. They are said to be “cyclable”, depending on the technology they can endure from 500 to 1500 charge-discharge cycles. The lead plates are thick and consume water. DIN designation: EPzS, British EPzB, nominal density of the electrolyte 1.28 to 1.30
Stationary batteries, a slow discharge variant. They are designed for reduced maintenance, therefore very little water supply. Two types in this category, those manufactured to provide backup energy, important in a short time (telecommunications, inverters, etc.) and those that accept deep discharge charge cycles (80%), solar energy, wind power, isolated sites. DIN designation: OPzV, OPzS, Solar suffix for the deep discharge type, often lower nominal electrolyte density, 1.24, but higher quantity of electrolyte. For equal capacity, the volume of batteries with lower density electrolyte is higher.
Three technologies.
With lead open, access is possible to compensate for the loss of water by adding liquid. A closed start-up variant exists, it is said to be "maintenance-free", a certain amount of water is provided to compensate for the inevitable losses, it is almost planned obsolescence. These open batteries are available in start-up, slow discharge (traction), stationary and solar versions.
AGM (Absorbed Glass Mat). The electrolyte is absorbed in a kind of fiberglass blotter (boro silicate). Their internal resistance is low and suitable for starting and have a certain ability to cycle. The gaseous releases of oxygen and hydrogen recombine in water. They are waterproof and maintenance free. A safety valve allows degassing to prevent accidental pressurization of the battery. VRLA: Valve Reguled Lead Acid
Gel. The electrolyte is always sulfuric acid and silica gel. Their internal resistance is higher and must be charged and discharged slowly with excellent cycling ability, withstand high temperatures well. Not very suitable for starting. They are waterproof and maintenance free. They are also VRLA.
These three battery technologies are regenerable!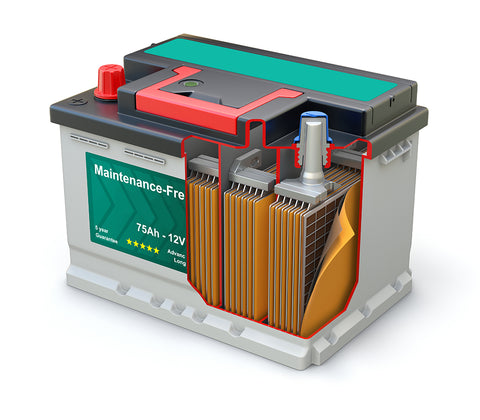
Two phases. At the start of charging, the current gently increases the battery voltage (the so-called absorption phase). After a threshold, 2.4V (floating phase) for a 2V cell, 14.4V for a 12V battery, there is electrolysis of the water therefore loss of liquid, the battery "boils". Charging therefore continues at an imposed charge voltage of 2.4V per cell. An equalization phase is desirable from time to time at 2.6V per element of 2V, at low intensity to avoid overheating, but only with open batteries, there is water loss, which must be compensated by adding demineralized water. To avoid this loss, on closed batteries (gel, AGM, and maintenance-free) it is necessary that the charger at the end of charging limit the voltage to less than 2.4V per cell. But sending 2.4V from the start would induce too large charge intensities, destructive for the battery. Choosing the right charger guarantees the durability of the batteries.
What you must remember.
Top-ups are only done with demineralized water, never acid.
With maintenance-free, AGM and gel batteries, use a specific charger, never overcharge them, the battery will dry out and be permanently lost.
Choose the right type of battery, starting, slow discharge (cyclable), stationary, or stationary cyclable (solar) depending on use.
Carefully monitor the electrolyte level in open starter and traction batteries, which is less critical with stationary and solar batteries that consume less water. Add water only on a charged battery. The volume naturally increases during charging.
Fully charge your batteries, even if it means slightly overcharging the open starter and traction or stationary batteries at the cost of easily compensable water loss.
What not to do, precautions.
On a discharged open lead battery, put a minimum level of the electrolyte if necessary, by adding demineralized water, until covering the plates, no more. The volume of electrolyte naturally increases during charging, with the risk of loss of acid which would spill out of its container. At the end of the charge, top up the level if necessary, always with demineralized water.
Never add sulfuric acid to a battery. If the electrolyte density is too low after a full charge, and equalization charge, this is a sign of sulfation. Instead, consider regeneration. The only case where the addition of acid is desirable is after a loss of electrolyte by overflow for example. Acid does not evaporate only water can, its electrolysis is the second cause of loss of electrolyte level.
Never pour water into acid, there is instant heating and there is a great risk of corrosive and dangerous splashes. Handle acids only protected by an apron, gloves, mask, boots and all other necessary and suitable personal protective equipment (PPE). All this resistant to corrosive chemicals.
The batteries deliver electric current, with the inherent risks of electrification. Only authorized and protected persons are able to operate in an environment where electric storage batteries are present.
Batteries must operate in a ventilated environment, the release of highly explosive oxygen and hydrogen is normal and dangerous. Beware of flames, do not smoke.
JUNLEE Group is an integrated full power energy factory that specializes in Uninterruptible Power Supply (UPS), Lead-Acid Battery, Battery pack, EV battery, Energy Storage Battery, Energy storage power station, Power pack Gel battery, PV Inverter and Solar system.
Production capacity reach 200000 KVaH per month. Products apply to Electric vehicles,electric mobility, solar & wind energy storage system, UPS, backup power, telecommunication, medical equipment and lighting.
JUNLEE sets up "Power research center" with more High-tech products.More than 100 engineers provided in-time and efficient one-stop solutions.
They mission strives to bring green power to the world.
To learn more about Li-ion batteries, please refer to https://www.junleepower.com/

