How to Meet Field Integrity and Airworthiness
During an external pre-flight inspection before takeoff, the pilot notices heavy white smoke billowing from the cargo hold of the Boeing 737 and declared “Mayday.” Emergency crews arrived and uncovered 28 batteries in a checked transit case, 6–8 of which had been destroyed by fire. The report said that an electrical short in a battery started the fire after the passenger declared that no batteries were in the transit case. Under civil aviation laws, passengers failing to declare dangerous goods face penalties of up to 7 years in prison. Shipping lithium batteries are subject to the Transportation of Dangerous Goods Act.
| The media is quick to report lithium-ion events, and for good reason. The Samsung Note 7 was banned on flights because of potential fire hazard; e-cigarettes catch fire, electric hover boards have been recalled, and in 2006 a one-in-200,000 breakdown triggered a recall of almost six million lithium-ion packs. Furthermore, the grounding of the Boeing 787 Dreamliner in 2012/2013 was due to a failed Li-ion battery. These mishaps occurred after the batteries in question passed rigorous UN 38.3 testing, the standard responsible for transporting lithium batteries under the United Nations and department of transport organization. |
 UN 38.3 Transport of Dangerous Goods is ruled by the United Nations |
Lithium-ion is safe but with millions of industrial and consumer applications, failures will happen. Microscopic metal particles coming in contact with other parts in the battery cell caused the Sony recall. Battery manufacturers strive to minimize the presence of such particles; however, assembly techniques make the elimination of such dust a challenge. Modern cells with ultra-thin separators of 21µm (21-thousandth of an mm) are more susceptible to impurities than older designs with a heavier separator and lower ampere-hour (Ah). While the classic 1,350mAh in the 18650 cell could tolerate a nail penetration; the energy-dense 3,400mAh of today turns into a firework when performing the same test. The 18650 is a standardized Li-ion cell introduced in the early 1990s of 18mm in diameter and 65mm in length.
There are two types of battery failures. One is a one-in-10-million incident involving a manufacturing flaw that mostly leads to a recall. The second is a rare random event alike being hit by a meteor. The reasons for such failures are hard to find as the damaged cell cannot be reconstructed. Possible causes are charging at sub-freezing temperature, high heat, excessive vibration and/or repeated heavy loading. Just talk to a hobbyist, a hover board race driver or a drone operator; they are familiar with these stresses.
Most battery failures leading to disintegration start with a mild electrical short that goes unnoticed. The battery performs normally and the user is unaware of a pending breakdown. This differs from a faulty structure that shows stress marks before falling. Structural anomalies are well documented and the failure threshold is known. In this respect, the battery behaves more like a black box with a mind of its own. Organizations such as NRTL, ANSI, UL, IEEE are beginning to study battery applications and mandate correct uses. A heavy truck will receive a long-lasting engine rather than a souped-up engine from a sports car of same horsepower.
Battery failures are also being caused by electrode deflection or abnormal weld spots, as was the case with the Samsung Note 7 smartphone. Fast charging at cold temperatures promotes dendrite formation, so does storing Li-ion below 1.5V/cell for more than a week. These stresses may lead to elevated self-discharge and an electrical short by building up heat spots that weakens the separator.
During thermal runaway, Li-ion vents, the temperature quickly rises to 500°C (932°F), at which point the cell catches fire or explodes. This is known as “venting with flame; “rapid disassembly” is the preferred term in the battery industry. The exhausting gases are carbon dioxide and carbon monoxide, as well as gases formed by the vaporizing electrolyte.
Protection Circuits
Because batteries can release high energies, a safety mechanism is required that prevents damage if a short is applied. The most basic device is a fuse that opens at high current. Some fuses open permanently and render the battery useless; others are more forgiving and reset. The positive thermal coefficient (PTC) is a re-settable device that creates high resistance on excess current and reverts back to normal.
Further layers of safeguards are solid-state switches that disconnect the battery when current or voltage readings rise above a set threshold. Current limitations are set according to the cell’s Ah rating; voltage limitations engage when exceeding 4.3V/cell on charge and 2.2V/cell on discharge of a Li-ion cell. (Other settings may apply.) Some cells are disabled if the temperature rises to 90ºC and/or the pressure exceeds a set limit. All switching devices have a residual resistance that causes a slight increase in overall battery resistance and a decrease in maximum current flow.
The protection circuit shields the battery from outside aggressions, but it is ineffective to stop an internal thermal runaway once in progress. Like a nuclear meltdown, the battery feeds on its own energy, however melting separators that are built into some advanced cells inhibiting ion flow at high temperature will slow the process down. The batteries recalled in 2006, as well as the main-ship battery in the Boeing 787 and batteries in other incidents passed all regulatory safety requirements, yet failed under normal use and with proper protection circuits in place.
Batteries entering a hazardous area must be intrinsically safe. This applies to mobile phones, two-way radios, laptops, cameras, flashlights, gas detectors, medical instruments, including devices operated with primary 9V, AA and AAA cells. Regulated under IEC 60079, a circuit built into the packs or device limits the current to prevent an electrical spark from forming that could ignite gases in oil refineries or chemical plants, as well as dust at grain elevators or textile mills. Certification in North America is by Factory Mutual, UL, CSA and others; Europe is under ATEX and most accept the IEC 60079 standard.
Building a Lithium-ion Pack
Building a Li-ion battery pack begins by estimating voltage, current and runtime requirements. Some consumer products go for a slim profile and the choice is a prismatic or pouch cell. If space allows, the 18650 provides the lowest cost and best performance in terms of specific energy, safety and durability.
The early Li-ion battery was considered fragile and unsuitable for high loads. Today, lithium-based systems stand shoulder-to-shoulder with robust nickel and lead chemistries. When building a Li-ion pack, two distinct types must be reviewed.
The Energy Cell is built for maximum capacity to provide long runtimes. The Panasonic NCR18650B Energy Cell in Figure 1 has a very high capacity when discharged at 0.5C (red line) but is less enduring at 2C (purple) at twice the rated current. Rather than delivering 3,200mAh to the 3V/cell end discharge line, the Energy Cell delivers only 2,300mAh (red circle); 28 percent less than specified.
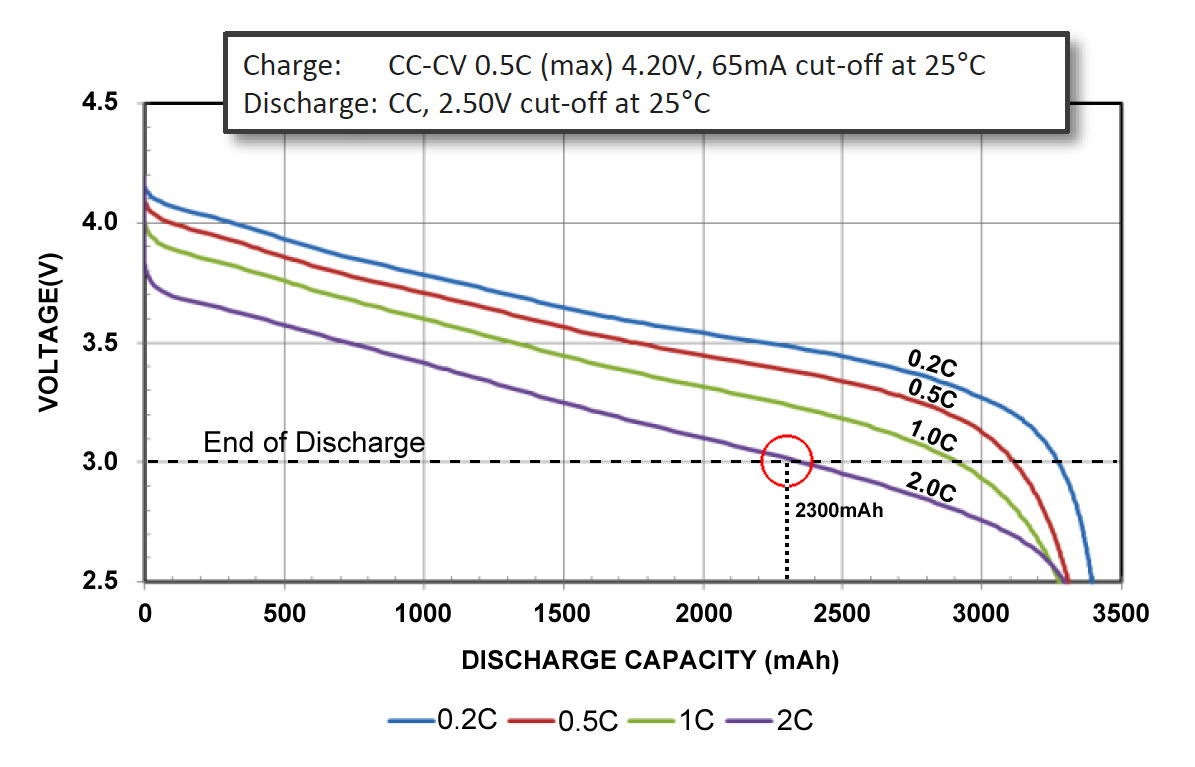 |
Figure 1: Discharge characteristics of NCR18650B Energy Cell by Panasonic in 18650 package. The 3,200mAh Energy Cell is discharged at 0.2C, 0.5C, 1C and 2C. The circle at the 3.0V/cell line marks the end-of-discharge point at 2C. Cold temperature losses: 25°C (77°F) = 100% 0°C (32°F) = ~83% –10°C (14°F) = ~66% –20°C (4°F) = ~53% Source: Panasonic |
The Panasonic UR18650RX Power Cell in Figure 2 has a moderate capacity of 1.95Ah but offers excellent load capabilities. A 10A (5C) discharge has minimal capacity loss at the 3.0V end of discharge voltage. This is ideal for applications requiring heavy load current, such as power tools. Although 40 percent less in capacity than the Energy Cell, the Power Cell also offers superior performance at cold temperatures.
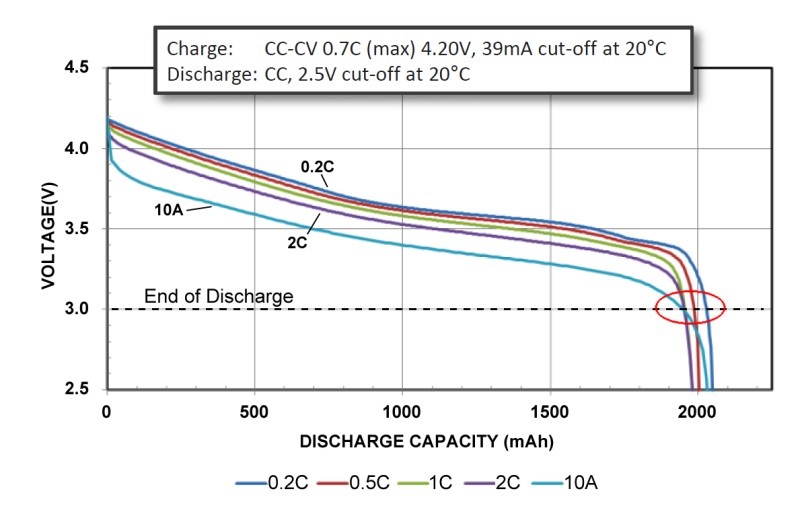 |
Figure 2: Discharge characteristics of UR18650RX Power Cell by Panasonic in 18650 package. The 1950mAh Power Cell is discharged at 0.2C, 0.5C, 1C and 2C and 10A. All reach the 3.0V/cell cut-off line at about 2000mAh. The Power Cell has a moderate capacity but delivers high current. Cold temperature losses: 25°C (77°F) = 100% 0°C (32°F) = ~92% –10°C (14°F) = ~85% –20°C (4°F) = ~80% Source: Panasonic |
The finished battery must undergo electrical and mechanical tests to meet the Manual of Tests and Criteria Recommendations according to UN 38.3 for the transportation of dangerous goods. These rules harmonize with Federal Aviation Administration (FAA), the US Department of Transport (DOT) and the International Air Transport Association (IATA). The certification applies to primary and secondary lithium cells and batteries and must meet these requirements:
T1 – Altitude Simulation: Low pressure simulating unpressurized cargo hold at 15,000 meters.
T2 – Thermal Test: Temperature extremes by keeping batteries for 6h at -40°C and +75°C.
T3 – Vibration: Test simulates vibration during transportation at 7Hz to 200Hz for up to 3 hours.
T4 – Shock: Test simulates vibration during transportation at given G-forces relating to battery size.
T5 – External Short Circuit: With fusing, apply a short circuit at 50°C. Case cannot exceed +170°C.
T6 – Impact: >20mm cylindrical cell tested for impact; all <20mm cell types tested for crushing.
T7 – Overcharge: Charge at twice the recommended current for 24 hours (secondary batteries only)
T8 – Forced Discharge: Same as T7, forced discharge with primary and secondary cells.
Authorized laboratories performing the tests require a pool of 24 batteries consisting of 12 new samples and 12 that have been cycled 50 times. The batteries must pass the tests without causing harm of involving disassembly, rupture of fire within 6 hours of the test, but the packs may perish during testing. IATA wants to ensure that the batteries in question are airworthy and have field integrity; cycling the packs 50 times before the test adds some reality.
The high certification cost of $10,000 to $20,000 discourages small manufacturers from using Li-ion for low-volume products and entrepreneurs may choose nickel-metal-hydride as this chemistry does not require the same level of testing. Obsolescence of an existing lithium cell is another issue that adds to cost. A recertification of the back is needed even if the new cells are a direct replacement. Regulators say that cell approval cannot be transferred to the pack because the safety confirmation is on a finished product and not the components.
What to Do when a Battery Overheats
If a Li-ion battery overheats, hisses or bulges, immediately move the device away from flammable materials and place it on a non-combustible surface. If at all possible, remove the battery and put it outdoors to burn out. You may also put the device outside and keep it there of a least 6 hours.
A small Li-ion fire can be handled like any other combustible fire. For best result use a foam extinguisher, CO2, ABC dry chemical, powdered graphite, copper powder or soda (sodium carbonate). Halon is also used as fire suppressant.
FAA instructs flight attendants to use water or soda pop to extinguish a fire in the cabin. Water-based products are most readily available and are appropriate since Li-ion contains very little lithium metal that reacts with water. Water also cools the adjacent area and prevents the fire from spreading. Research laboratories and factories use water to extinguish small Li-ion fires.
A large Li-ion fire, such as an EV, may need to burn out as water is ineffective. Water with copper material can be used, but this may not be available and is costly for fire halls. When encountering a fire with a lithium-metal battery, only use a Class D fire extinguisher. Lithium-metal contains lithium that reacts with water and makes the fire worse. Only use the Class D fire extinguisher on lithium fires.
| CAUTION | Do not use a Class D fire extinguisher to put out other types of fires; make certain regular extinguishers are also available. With all battery fires, allow ample ventilation while the battery burns itself out. |
During a thermal runaway, heat from the failing cell in a battery pack may propagate to the next cell, leading to thermally instability. A chain reaction can occur in which each cell disintegrates on its own timetable. A pack can thus be destroyed in a few seconds or over several hours as each cell is consumed. Packs should include dividers to protect a failing cell from spreading to the neighboring one. Mobile phones run on a single Li-ion cell whereas laptop batteries have several cells connected in series and parallel to achieve the needed voltage and Ah rating.
About the Author
Isidor Buchmann is the founder and CEO of Cadex Electronics Inc. For three decades, Buchmann has studied the behavior of rechargeable batteries in practical, everyday applications, has written award-winning articles including the best-selling book “Batteries in a Portable World,” now in its fourth edition. Cadex specializes in the design and manufacturing of battery chargers, analyzers and monitoring devices

